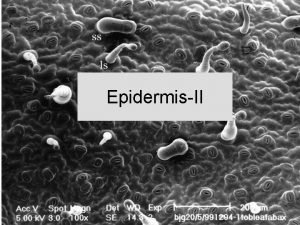
Plant water regime Mechanisms of stomata movements General





- Slides: 28

Plant water regime • Mechanisms of stomata movements – – – General characteristics Response of stomata to irradiance Response of stomata to water stress Response of stomata to air humidity Response of stomata to CO 2 Response of stomata to other factors

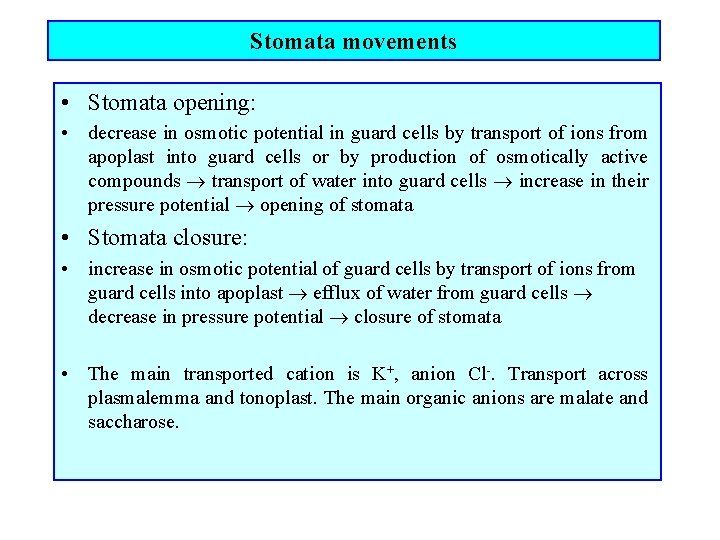
Stomata movements • Stomata opening: • decrease in osmotic potential in guard cells by transport of ions from apoplast into guard cells or by production of osmotically active compounds transport of water into guard cells increase in their pressure potential opening of stomata • Stomata closure: • increase in osmotic potential of guard cells by transport of ions from guard cells into apoplast efflux of water from guard cells decrease in pressure potential closure of stomata • The main transported cation is K+, anion Cl-. Transport across plasmalemma and tonoplast. The main organic anions are malate and saccharose.

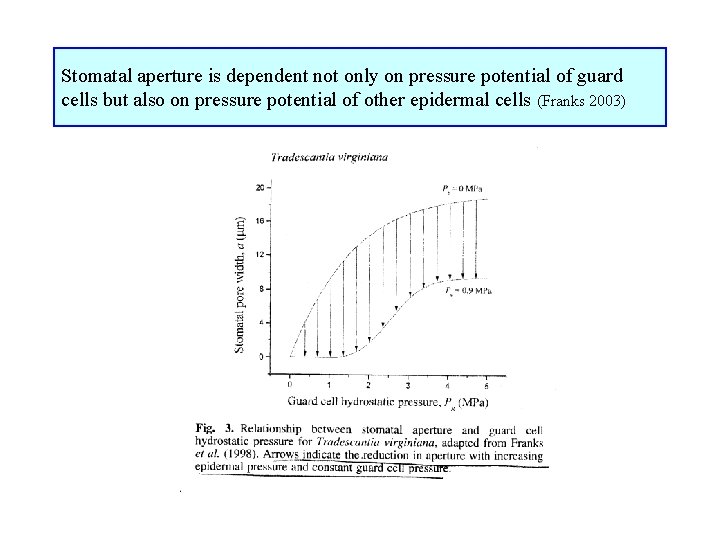
Stomatal aperture is dependent not only on pressure potential of guard cells but also on pressure potential of other epidermal cells (Franks 2003)

Transport of ions across membranes Usually by different channels, often not the same for influx and efflux. Transport rate is dependent on 1) membrane potential 2) concentration of transported ion 3) concentration of other ions or molecules 4) structure modifications due to phosphorylation or dephosphorylation of channel proteins 5) mechanical strength

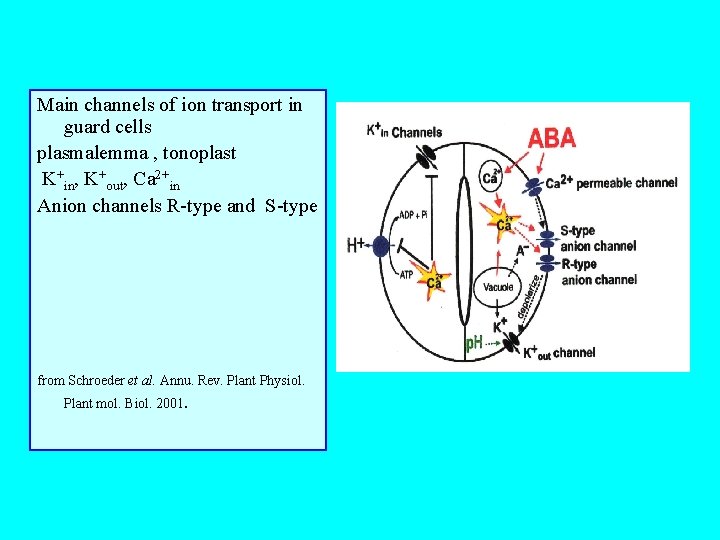
Main channels of ion transport in guard cells plasmalemma , tonoplast K+in, K+out, Ca 2+in Anion channels R-type and S-type from Schroeder et al. Annu. Rev. Plant Physiol. Plant mol. Biol. 2001 .

Response of stomata to irradiance the main factor inducing stomata opening • Blue and red radiation can influence directly guard cells • Receptors of blue radiation are: – phytochromes or flavoproteins (phototropins) mostly situated in plasmalemma – zeaxanthin in chloroplasts (interaction between irradiance and CO 2 concentration) • Possible mechanism: – activation of ATPase by phosphorylation on C-terminal active transport of H+ into apolast membrane polarization (electric potential about -120 m. V) activation of membrane channels for transport of K+ into guard cells (K+in) usually accompanied by Cl-. • Sensitivity of guard cells to blue radiation is much higher than to red radiation which is important for opening of stomata in the early morning

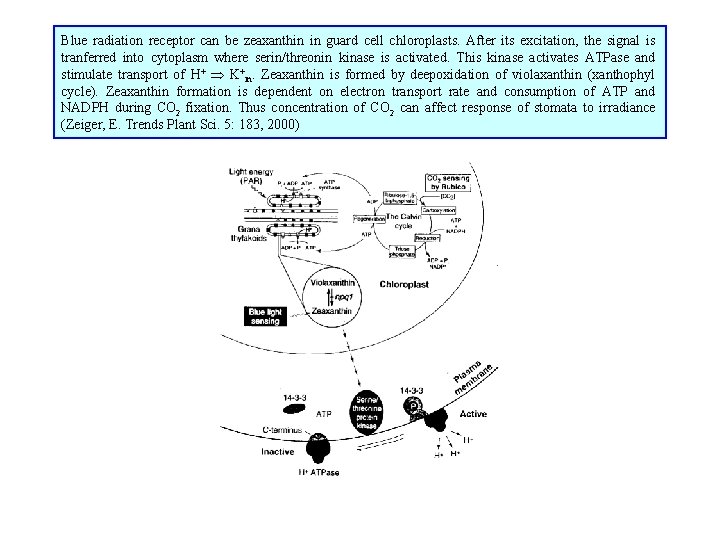
Blue radiation receptor can be zeaxanthin in guard cell chloroplasts. After its excitation, the signal is tranferred into cytoplasm where serin/threonin kinase is activated. This kinase activates ATPase and stimulate transport of H+ K+in. Zeaxanthin is formed by deepoxidation of violaxanthin (xanthophyl cycle). Zeaxanthin formation is dependent on electron transport rate and consumption of ATP and NADPH during CO 2 fixation. Thus concentration of CO 2 can affect response of stomata to irradiance (Zeiger, E. Trends Plant Sci. 5: 183, 2000)

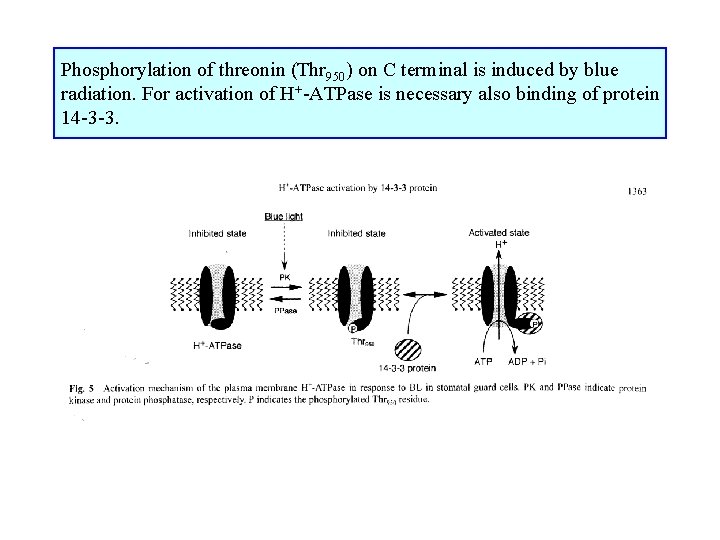
Phosphorylation of threonin (Thr 950) on C terminal is induced by blue radiation. For activation of H+-ATPase is necessary also binding of protein 14 -3 -3.

Effects of red radiation • Red radiation receptors are chloroplasts • Possible mechanisms: – induction of photosynthesis in guard cell chloroplasts – cyclic phosphorylation might be source of ATP – activation of PEPC (PEP from starch is carboxylated to oxalacetate, which is further reduced to malate). – accumulation of saccharose • Radiation can have indirect effect – it can stimulate photosynthesis in mesophyll cells and thus decrease internal CO 2 concentration.

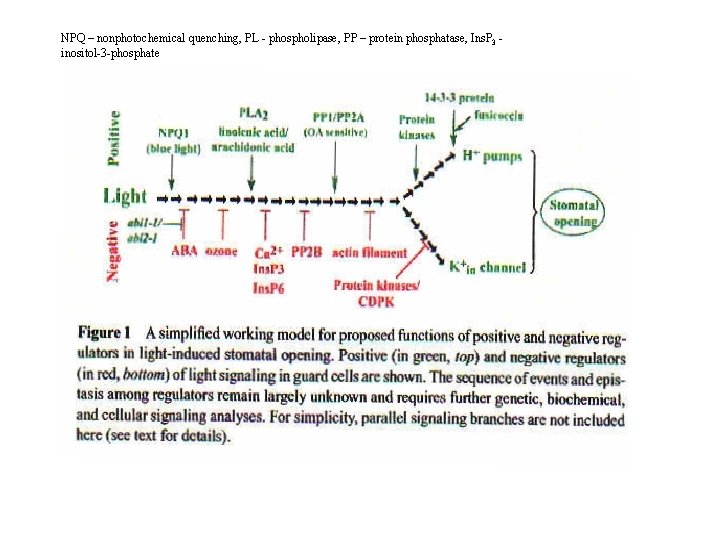
NPQ – nonphotochemical quenching, PL - phospholipase, PP – protein phosphatase, Ins. P 3 inositol-3 -phosphate

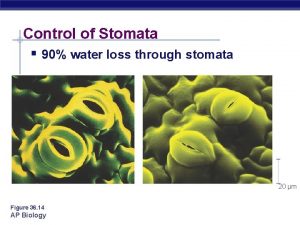
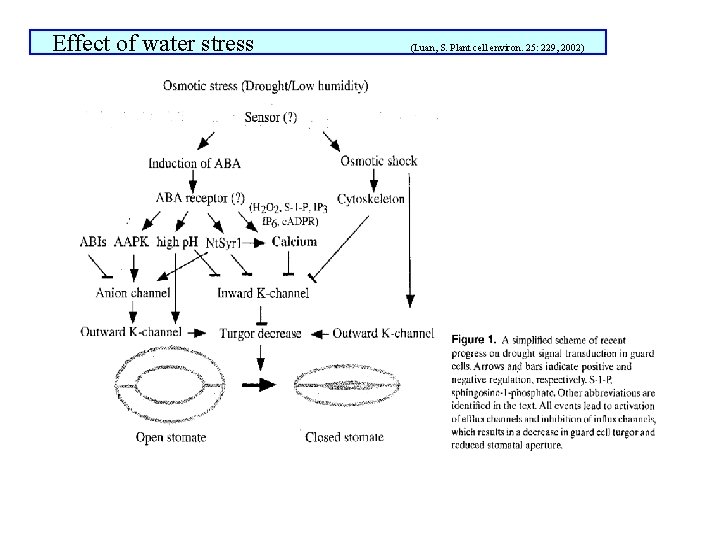
Response of stomata to water stress main factor inducing stomata closure Water deficit in leaf mesophyll occurs in consequence of low air humidity, low soil moisture, salinity, low soil temperature hydraulic signals (direct effects) : water content in guard cells water content in other epidermal cells pressure potential in xylem chemical signals (indirect effects): positive or negative: ABA, other phytohormones, mineral ions, polyamines, sfingosin-1 -phosphate, H 2 O 2, , NO Due to decrease in water content in apoplast under high transpiration rate ABA concentration in apoplast increases (at the same amount of ABA) and so apparently increases stomata sensitivity to ABA Transport of K+ in channels can be affected by mechanical strength on the membrane

Effect of water stress (Luan, S. Plant cell environ. 25: 229, 2002)

ABA is stress phytohormone and it enables root-to-shoot communication Synthesis in leaves as well as in roots, precursors are carotenoids • • • 9 -cis-violaxanthin or 9 -cis-neoxanthin xanthoxin (9 -cis-epoxycarotenoid dioxygenase; NCED). xanthoxin abscisic acid aldehyde (xanthoxin oxidase). abscisic acid aldehyde ABA (ABA aldehyde oxidase) Distribution xylem, phloem, apoplast and symplast, dependent on p. H. Degradation – to phaseic or dihydrophaseic acid Concentration of ABA in xylem about 10 nmol dm-3, under stress 3 mol dm-3. For regulation of stomata opening is important ABA concentration in apoplast around guard cells. ABA is released from leaf mesophyll cells or it is transported from roots in the form of free ABA or ABA conjugates (they can be converted back to free ABA by -glucosidase). ABA also increases hydraulic permeability in roots, affects gene expression, inhibits growth of shoots more than that of roots, stimulates leaf fall, plant ageing, etc.

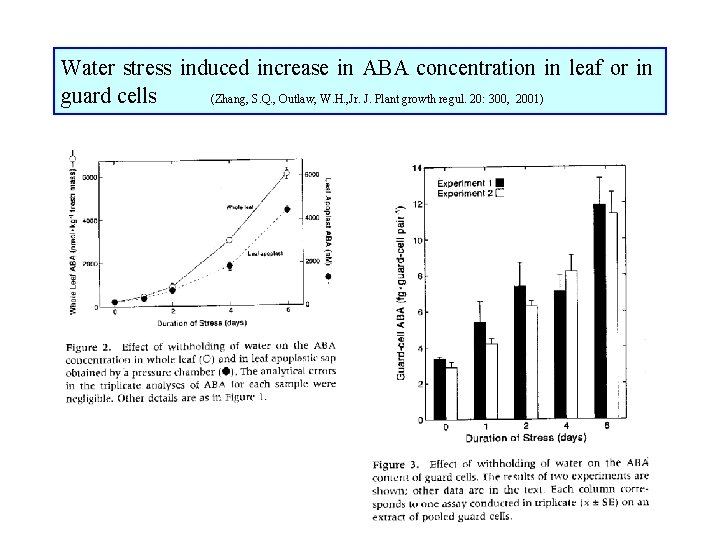
Water stress induced increase in ABA concentration in leaf or in guard cells (Zhang, S. Q. , Outlaw, W. H. , Jr. J. Plant growth regul. 20: 300, 2001)

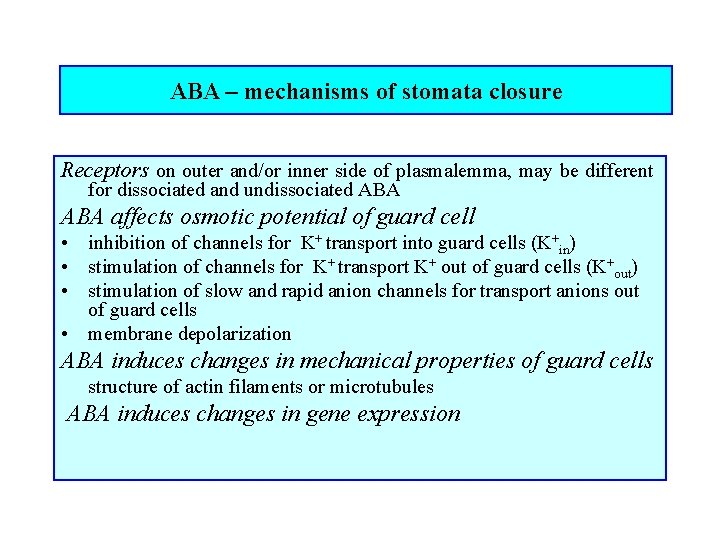
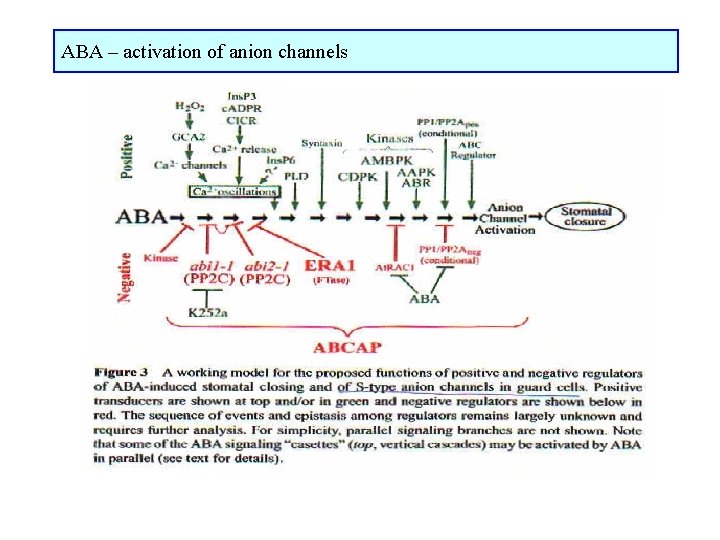
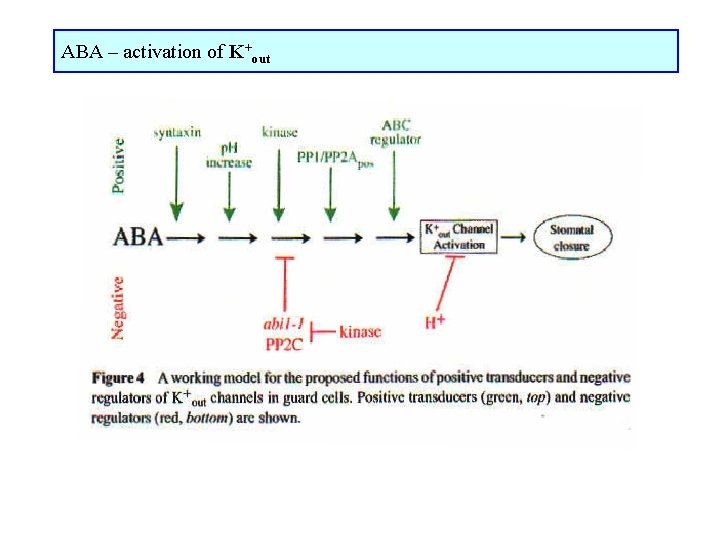
ABA – mechanisms of stomata closure Receptors on outer and/or inner side of plasmalemma, may be different for dissociated and undissociated ABA affects osmotic potential of guard cell • inhibition of channels for K+ transport into guard cells (K+in) • stimulation of channels for K+ transport K+ out of guard cells (K+out) • stimulation of slow and rapid anion channels for transport anions out of guard cells • membrane depolarization ABA induces changes in mechanical properties of guard cells structure of actin filaments or microtubules ABA induces changes in gene expression

Ca 2+ - second messenger Changes in Ca 2+ concentration are general intermediates in signalling pathways leading to stomata closure as well as to stomata opening Ca 2+ is transported either from apoplast or from storages in vacuoles by special channels Ca 2+ channels are activated by: • inositol-1, 4, 5 -phosphate, inositolhexakisphosphate, • cyclic adenosine-diphosphoribose (c. ADPR) • G-proteins phosphorylation and dephosphorylation of proteins, calmodulin changes in Ca 2+ oscillations Stomata closing: inhibition of K+in, , stimulation of K+out, , anion channels Stomata opening: Ca 2+ may activate Ca-dependent protein kinase which activate tonoplast channels for Cl-

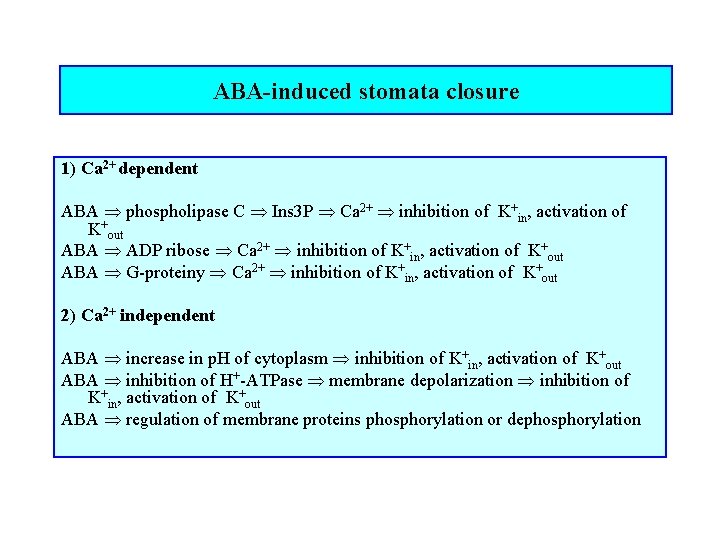
ABA-induced stomata closure 1) Ca 2+ dependent ABA phospholipase C Ins 3 P Ca 2+ inhibition of K+in, activation of K+out ABA ADP ribose Ca 2+ inhibition of K+in, activation of K+out ABA G-proteiny Ca 2+ inhibition of K+in, activation of K+out 2) Ca 2+ independent ABA increase in p. H of cytoplasm inhibition of K+in, activation of K+out ABA inhibition of H+-ATPase membrane depolarization inhibition of K+in, activation of K+out ABA regulation of membrane proteins phosphorylation or dephosphorylation


ABA – activation of anion channels

ABA – activation of K+out

Air humidity • • • Decrease in air humidity increased peristomatal transpiration decreased guard cell pressure potential stomata closure Decrease in air humidity increased transpiration decreased leaf water potential stomata closure Decrease in air humidity increased apoplast ABA concentration stomata closure

CO 2 concentration Response of stomata to CO 2 is in the dark as well as under irradiance, sensitivity under irradiance is higher Low CO 2 concentration stimulates stomata opening, high CO 2 concentration their closure Possible mechanisms: • a) increased ci decreased zeathin concentration in chloroplasts decreased ATPase activation less stomata opening • b) changes in Ca 2+ oscillation frequency (low CO 2 – higher frequency) • c) increased ci release of malate from mesophyll cell to apoplast activation of R-type anion channels stomata closure • d) increased ci increased malate production in guard cells leading to inhibition of H+ transport, membrane depolarization and stimulation of anion channels and K+out


Other phytohormones • • • Auxins – increase or decrease gs Cytokinins – increase or do not change gs Gibberellins – increase or decrease gs Jasmonates - decrease gs Brassinosteroids - decrease gs Ethylene - ? ? • • • Mechanisms similar to those of ABA: affection of Ca 2+ activation or inhibition of ATPase Interactions among phytohormones

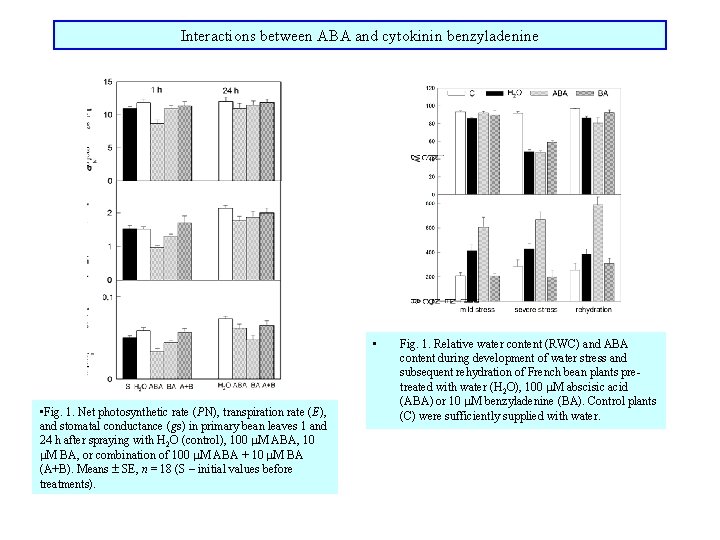
Interactions between ABA and cytokinin benzyladenine • • Fig. 1. Net photosynthetic rate (PN), transpiration rate (E), and stomatal conductance (gs) in primary bean leaves 1 and 24 h after spraying with H 2 O (control), 100 M ABA, 10 M BA, or combination of 100 M ABA + 10 M BA (A+B). Means SE, n = 18 (S – initial values before treatments). Fig. 1. Relative water content (RWC) and ABA content during development of water stress and subsequent rehydration of French bean plants pretreated with water (H 2 O), 100 M abscisic acid (ABA) or 10 M benzyladenine (BA). Control plants (C) were sufficiently supplied with water.

Other factors • • • Pollutants SO 2, NO 2, O 3 – at high concentration usually increased stomata opening, transpiration rate and so increase risk of wilting Fusiccocin (fytotoxin produced by Fusicoccum amygdali) – induces stomata opening by activation of proton pump H 2 O 2, NO – signal molecules for different stresses

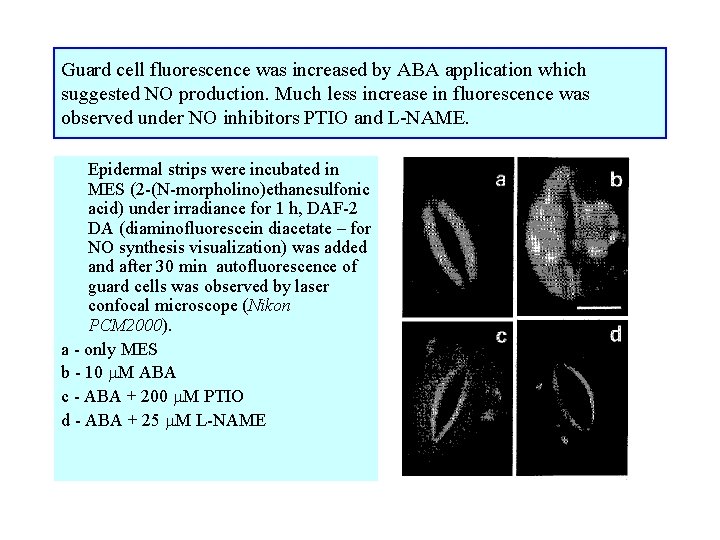
Guard cell fluorescence was increased by ABA application which suggested NO production. Much less increase in fluorescence was observed under NO inhibitors PTIO and L-NAME. Epidermal strips were incubated in MES (2 -(N-morpholino)ethanesulfonic acid) under irradiance for 1 h, DAF-2 DA (diaminofluorescein diacetate – for NO synthesis visualization) was added and after 30 min autofluorescence of guard cells was observed by laser confocal microscope (Nikon PCM 2000). a - only MES b - 10 M ABA c - ABA + 200 M PTIO d - ABA + 25 M L-NAME

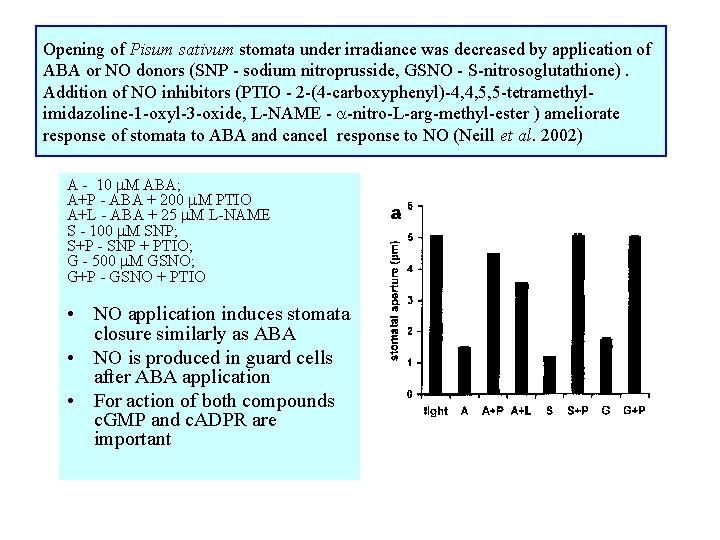
Opening of Pisum sativum stomata under irradiance was decreased by application of ABA or NO donors (SNP - sodium nitroprusside, GSNO - S-nitrosoglutathione). Addition of NO inhibitors (PTIO - 2 -(4 -carboxyphenyl)-4, 4, 5, 5 -tetramethylimidazoline-1 -oxyl-3 -oxide, L-NAME - -nitro-L-arg-methyl-ester ) ameliorate response of stomata to ABA and cancel response to NO (Neill et al. 2002) A - 10 M ABA; A+P - ABA + 200 M PTIO A+L - ABA + 25 M L-NAME S - 100 M SNP; S+P - SNP + PTIO; G - 500 M GSNO; G+P - GSNO + PTIO • NO application induces stomata closure similarly as ABA • NO is produced in guard cells after ABA application • For action of both compounds c. GMP and c. ADPR are important
 Only water vapor evaporates through stomata.
Only water vapor evaporates through stomata. Water and water and water water
Water and water and water water Axial movement arm
Axial movement arm Stomata process
Stomata process Stomata process
Stomata process Autotrophic nutrition equation
Autotrophic nutrition equation Bloody chlorophyll
Bloody chlorophyll Stomata in leaves
Stomata in leaves Stomata lab answers
Stomata lab answers Epidermis
Epidermis Stomata
Stomata Sel penutup pada stomata
Sel penutup pada stomata Tissue epidermis
Tissue epidermis Stomata synonym
Stomata synonym Uniserate stomata
Uniserate stomata Adaptation in plants
Adaptation in plants Lateral meristem
Lateral meristem Zona akar
Zona akar What are stomata
What are stomata Sunken stomata definition
Sunken stomata definition Stomata lab
Stomata lab Streamlike movements of water in the ocean called
Streamlike movements of water in the ocean called Plant introduction
Plant introduction Plant breeding for disease resistance
Plant breeding for disease resistance Plant introduction in plant breeding
Plant introduction in plant breeding Tronsmo plant pathology and plant diseases download
Tronsmo plant pathology and plant diseases download Tronsmo plant pathology and plant diseases download
Tronsmo plant pathology and plant diseases download Albugo eye
Albugo eye Transistor bipolaire regime dynamique
Transistor bipolaire regime dynamique