Physics Wave particle Duality Models of the Atom




- Slides: 17

Physics Wave particle Duality Models of the Atom

Waves Have a Particle Nature l Light can behave as particles of energy that possess kinetic energy and momentum l We see this when light strikes the surface of something – it transfers energy and momentum! l This was observed earlier in the century that when light above a minimum frequency value strikes the surface of specific metals, electrons are emitted from the metals l This is called the photoelectric effect – CLICK HERE view demo and do worksheet

Models of the Atom l Major Contributors: l Thomson (Lord Kelvin) early 1900’s l Rutherford 1920’s l Neils Bohr 2 years later! l Erwin Schrödinger - The Cloud Model

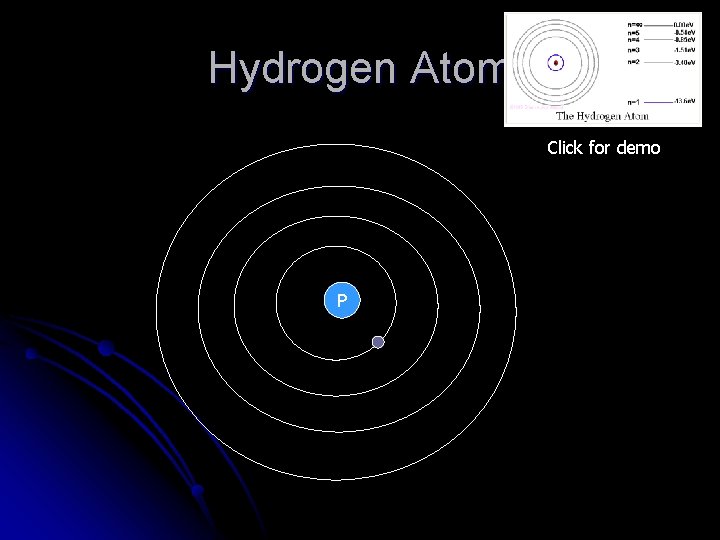
Bohr Model of the Hydrogen Atom l l Bohr proposed that electrons hold their positions and DO NOT spiral towards the nucleus Proposes (4) assumptions

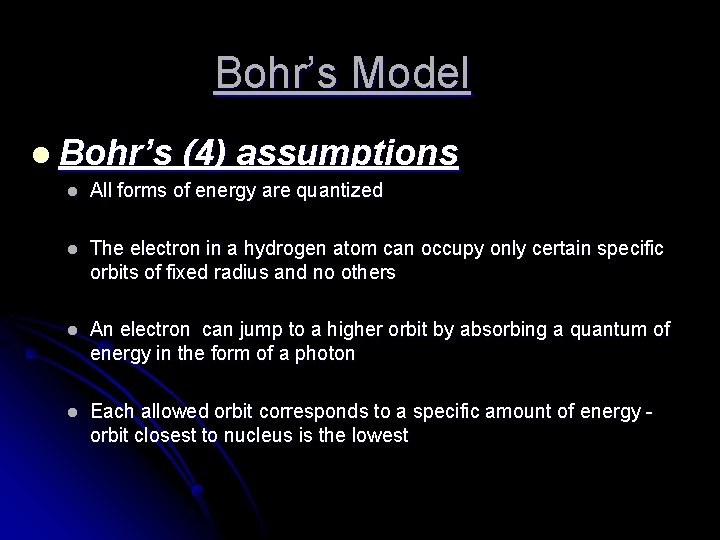
Bohr’s Model l Bohr’s (4) assumptions l All forms of energy are quantized l The electron in a hydrogen atom can occupy only certain specific orbits of fixed radius and no others l An electron can jump to a higher orbit by absorbing a quantum of energy in the form of a photon l Each allowed orbit corresponds to a specific amount of energy orbit closest to nucleus is the lowest

Hydrogen Atom Click for demo P

Emission (Bright-Line) Spectra l l When these frequencies are viewed in a spectroscope, the frequencies appear as a series of bright-lines against a dark background These are called Bright-Line Spectrum or Emission Spectrum

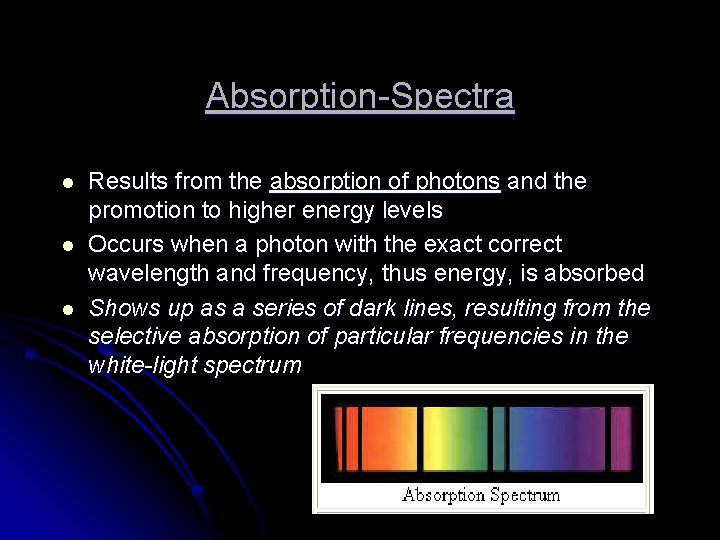
Absorption-Spectra l l l Results from the absorption of photons and the promotion to higher energy levels Occurs when a photon with the exact correct wavelength and frequency, thus energy, is absorbed Shows up as a series of dark lines, resulting from the selective absorption of particular frequencies in the white-light spectrum

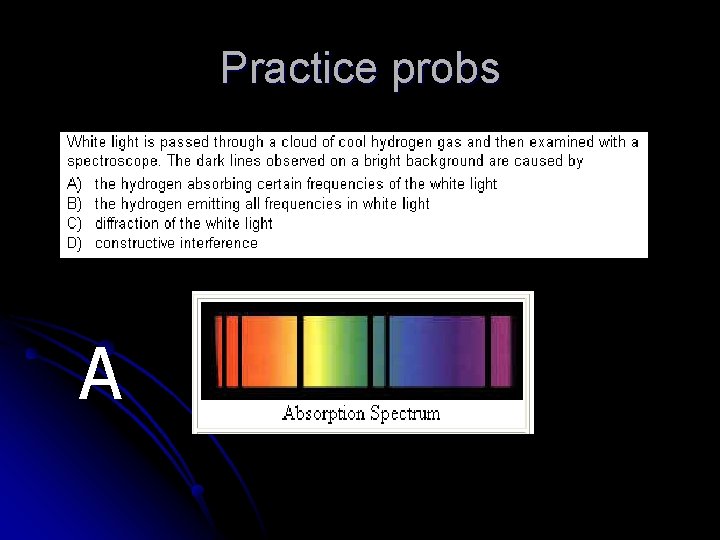
Practice probs A

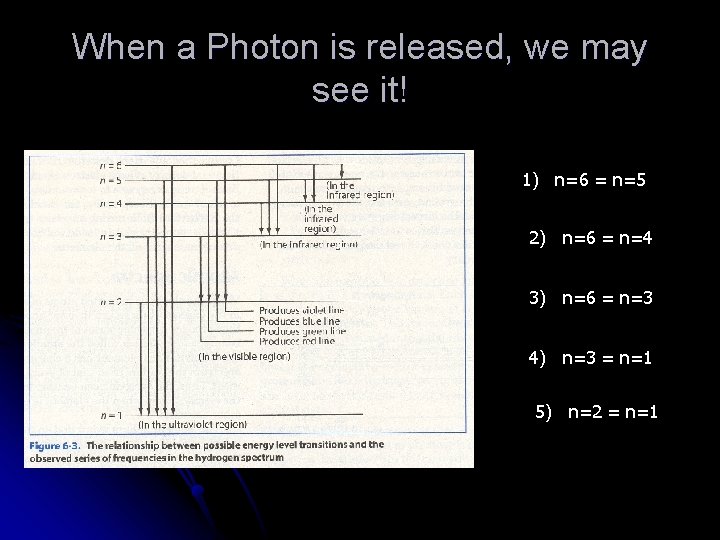
When a Photon is released, we may see it! 1) n=6 = n=5 2) n=6 = n=4 3) n=6 = n=3 4) n=3 = n=1 5) n=2 = n=1

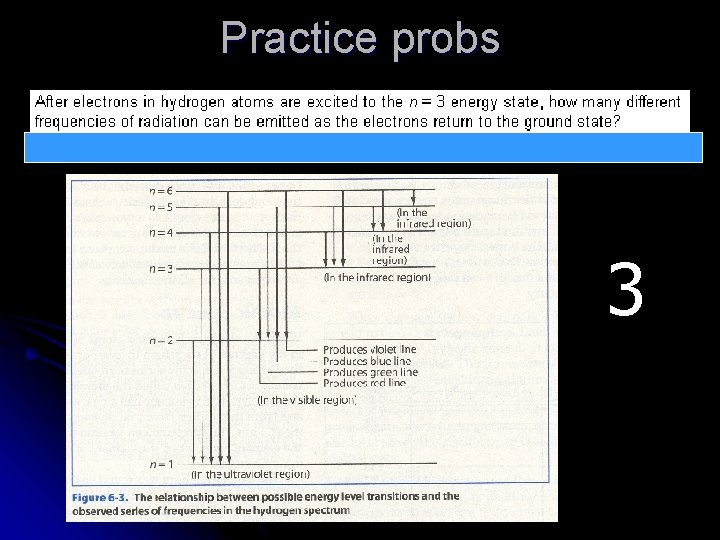
Practice probs 3

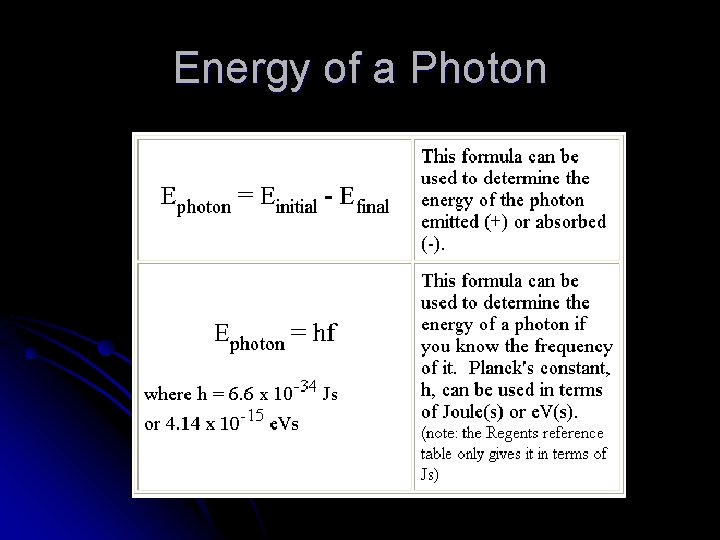
Energy of a Photon

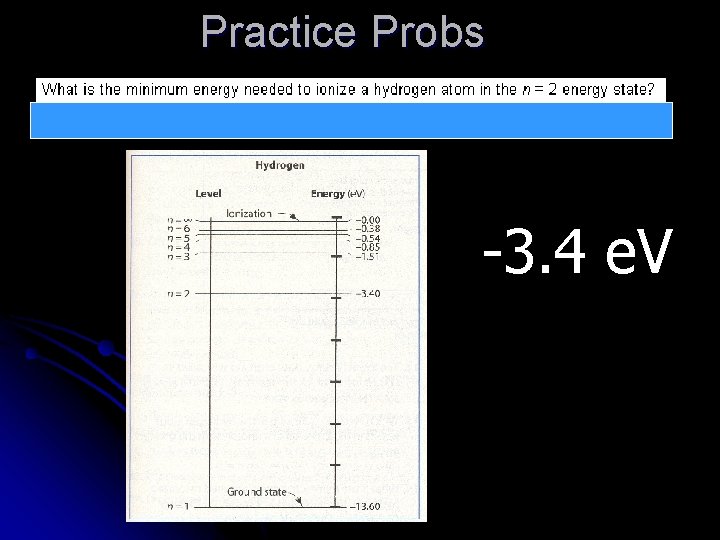
Practice Probs -3. 4 e. V

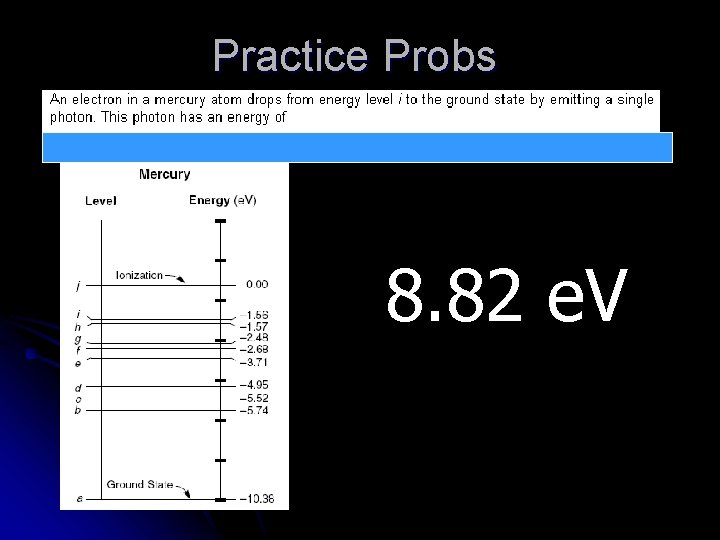
Practice Probs 8. 82 e. V

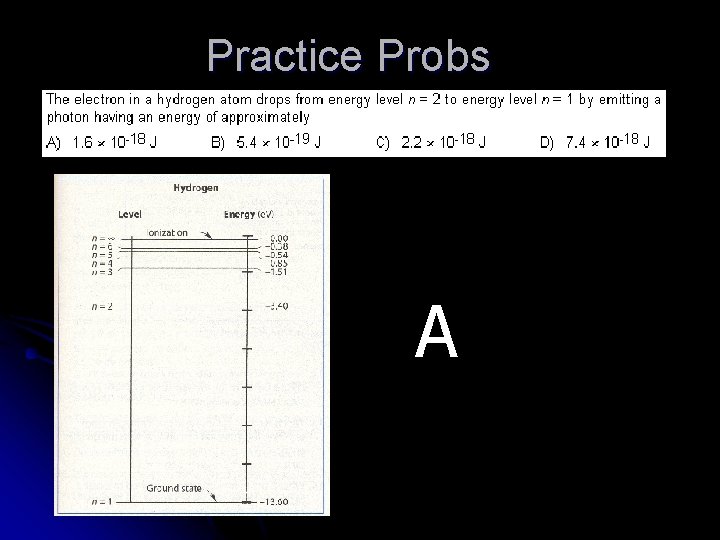
Practice Probs A

The Cloud Model Click above for Demo

Practice Prob A