Physics 151 Lecture 37 Todays Agenda l Topics






- Slides: 27

Physics 151: Lecture 37 Today’s Agenda l Topics çTemperature and Zeroth Law of Thermodynamics çTemperature scales çThermal expansion çIdeal gas Physics 151: Lecture 37, Pg 1

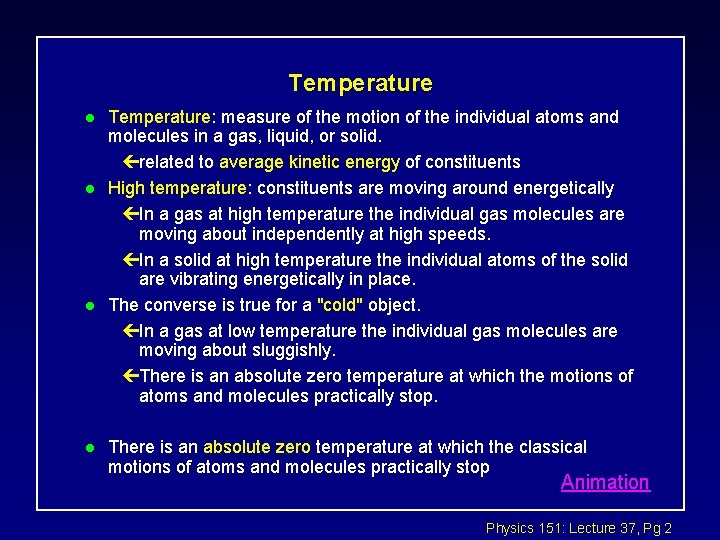
Temperature l l Temperature: measure of the motion of the individual atoms and molecules in a gas, liquid, or solid. çrelated to average kinetic energy of constituents High temperature: constituents are moving around energetically çIn a gas at high temperature the individual gas molecules are moving about independently at high speeds. çIn a solid at high temperature the individual atoms of the solid are vibrating energetically in place. The converse is true for a "cold" object. çIn a gas at low temperature the individual gas molecules are moving about sluggishly. çThere is an absolute zero temperature at which the motions of atoms and molecules practically stop. There is an absolute zero temperature at which the classical motions of atoms and molecules practically stop Animation Physics 151: Lecture 37, Pg 2

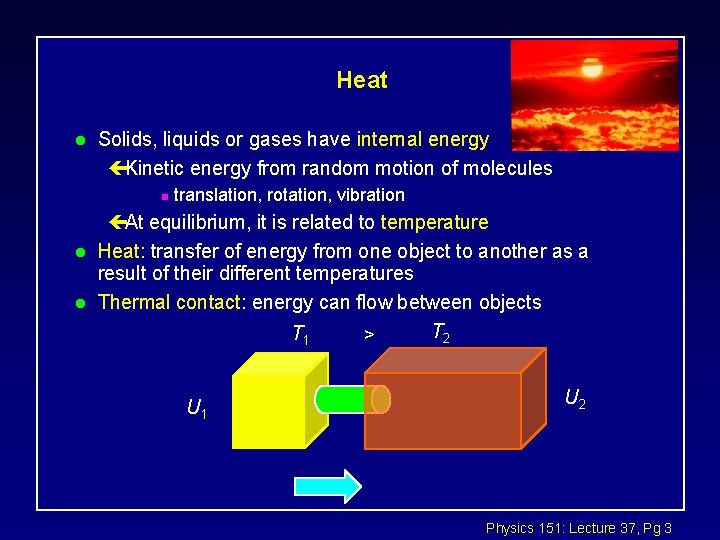
Heat l Solids, liquids or gases have internal energy çKinetic energy from random motion of molecules n l l translation, rotation, vibration çAt equilibrium, it is related to temperature Heat: transfer of energy from one object to another as a result of their different temperatures Thermal contact: energy can flow between objects T 2 T 1 > U 1 U 2 Physics 151: Lecture 37, Pg 3

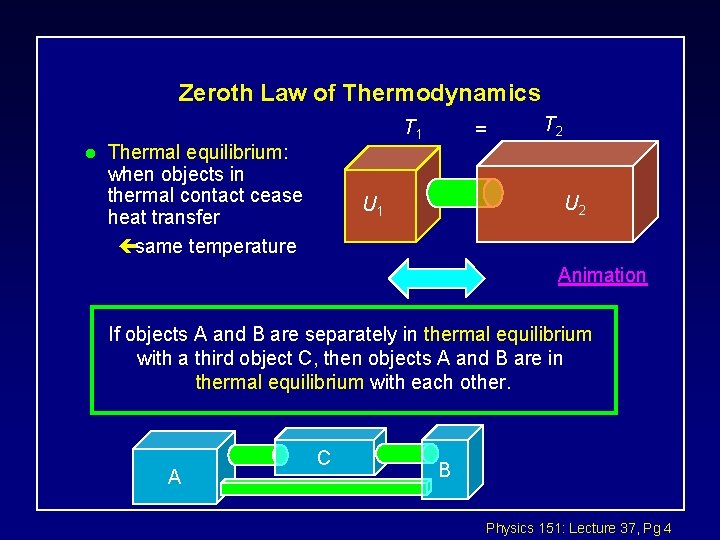
Zeroth Law of Thermodynamics l T 1 Thermal equilibrium: when objects in thermal contact cease heat transfer çsame temperature = T 2 U 1 Animation If objects A and B are separately in thermal equilibrium with a third object C, then objects A and B are in thermal equilibrium with each other. A C B Physics 151: Lecture 37, Pg 4

Thermometers l l A thermometer is a device that is used to measure the temperature of a system Thermometers are based on the principle that some physical property of a system changes as the system’s temperature changes These properties include: ç The volume of a liquid ç The dimensions of a solid ç The pressure of a gas at a constant volume ç The volume of a gas at a constant pressure ç The electric resistance of a conductor ç The color of an object A temperature scale can be established on the basis of any of these physical properties Physics 151: Lecture 37, Pg 5

Thermometer, Liquid in Glass l l A common type of thermometer is a liquid -in-glass The material in the capillary tube expands as it is heated The liquid is usually mercury or alcohol A thermometer can be calibrated by placing it in contact with some natural systems that remain at constant temperature Physics 151: Lecture 37, Pg 6

Thermometer, Celsius Scale l l A thermometer can be calibrated by placing it in contact with some natural systems that remain at constant temperature Common systems involve water ç A mixture of ice and water at atmospheric pressure » Called the ice point of water ç A mixture of water and steam in equilibrium » Called the steam point of water Celsius Scale : l l The ice point of water is defined to be 0 o C The steam point of water is defined to be 100 o C Physics 151: Lecture 37, Pg 7

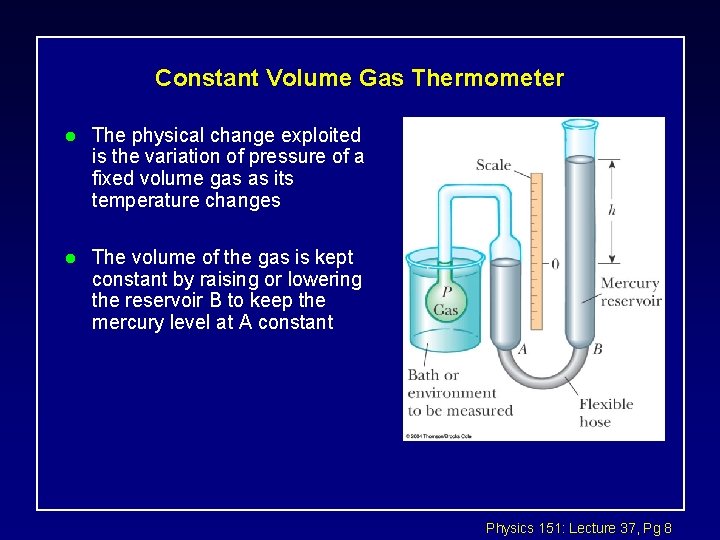
Constant Volume Gas Thermometer l The physical change exploited is the variation of pressure of a fixed volume gas as its temperature changes l The volume of the gas is kept constant by raising or lowering the reservoir B to keep the mercury level at A constant Physics 151: Lecture 37, Pg 8

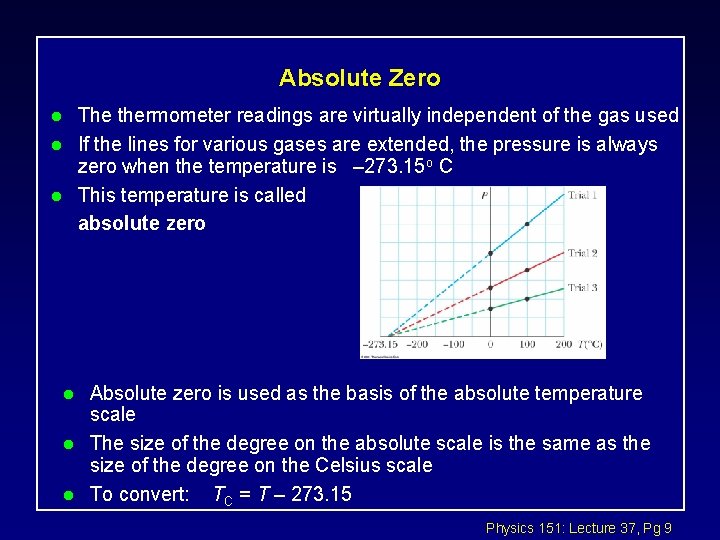
Absolute Zero The thermometer readings are virtually independent of the gas used If the lines for various gases are extended, the pressure is always zero when the temperature is – 273. 15 o C This temperature is called absolute zero l l l Absolute zero is used as the basis of the absolute temperature scale The size of the degree on the absolute scale is the same as the size of the degree on the Celsius scale To convert: TC = T – 273. 15 Physics 151: Lecture 37, Pg 9

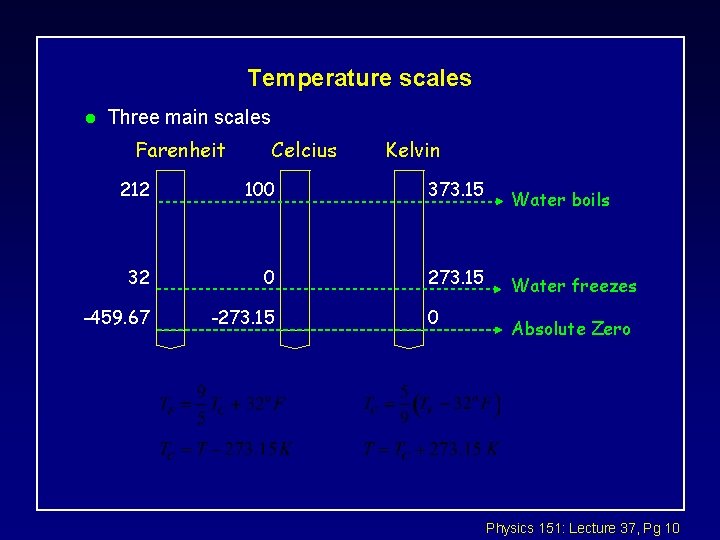
Temperature scales l Three main scales Farenheit Celcius Kelvin 212 100 373. 15 32 0 273. 15 -459. 67 -273. 15 0 Water boils Water freezes Absolute Zero Physics 151: Lecture 37, Pg 10

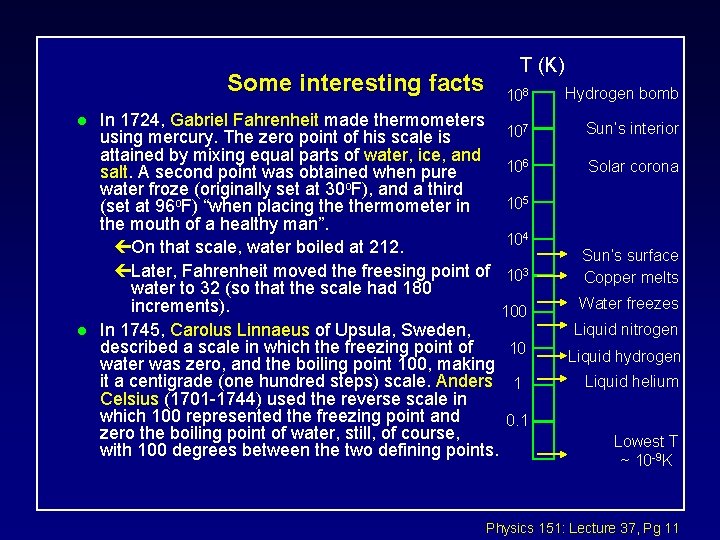
Some interesting facts l l T (K) 108 In 1724, Gabriel Fahrenheit made thermometers 107 using mercury. The zero point of his scale is attained by mixing equal parts of water, ice, and 106 salt. A second point was obtained when pure water froze (originally set at 30 o. F), and a third 105 (set at 96 o. F) “when placing thermometer in the mouth of a healthy man”. 104 çOn that scale, water boiled at 212. çLater, Fahrenheit moved the freesing point of 103 water to 32 (so that the scale had 180 increments). 100 In 1745, Carolus Linnaeus of Upsula, Sweden, described a scale in which the freezing point of 10 water was zero, and the boiling point 100, making it a centigrade (one hundred steps) scale. Anders 1 Celsius (1701 -1744) used the reverse scale in which 100 represented the freezing point and 0. 1 zero the boiling point of water, still, of course, with 100 degrees between the two defining points. Hydrogen bomb Sun’s interior Solar corona Sun’s surface Copper melts Water freezes Liquid nitrogen Liquid hydrogen Liquid helium Lowest T ~ 10 -9 K Physics 151: Lecture 37, Pg 11

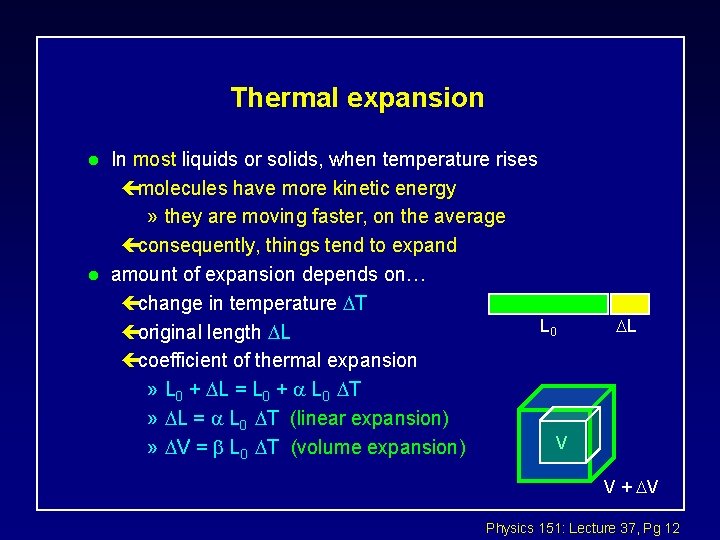
Thermal expansion l l In most liquids or solids, when temperature rises çmolecules have more kinetic energy » they are moving faster, on the average çconsequently, things tend to expand amount of expansion depends on… çchange in temperature T L 0 çoriginal length L çcoefficient of thermal expansion » L 0 + L = L 0 + L 0 T » L = L 0 T (linear expansion) V » V = L 0 T (volume expansion) L V + V Physics 151: Lecture 37, Pg 12

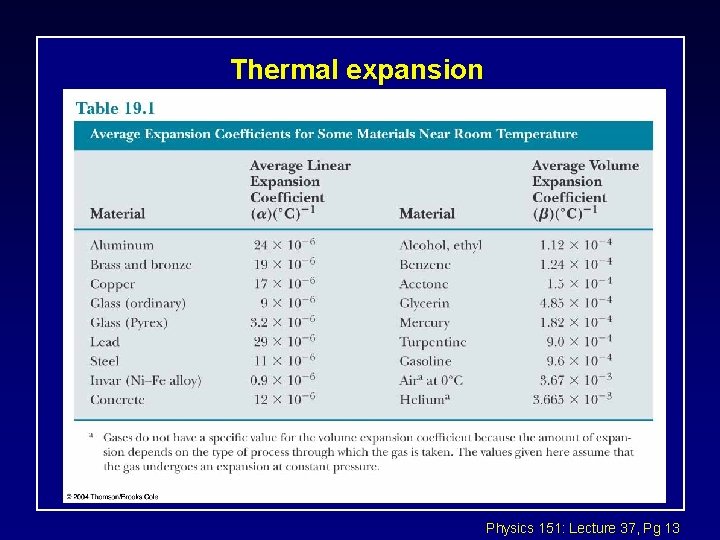
Thermal expansion Physics 151: Lecture 37, Pg 13

Bimetallic strip l l A bimetallic strip is made of two ribbons of dissimilar metals bonded together. Assume that the strip is originally straight. As they are heated, the metal with the greater average coefficient of expansion ( 2) expands more than the other, forcing the strip into an arc, with the outer radius having a greater circumference (as in the Fig). A compact spiral bimetallic strip is used in a home thermostat. Find the angle (Q) through which the free end of the strip turns when the temperature changes by 1°C for such bimetalic strip with the initial length L= 21. 0 cm and the separation of the centers of the Q strips ( r = r 2 - r 1 = 0. 410 mm). The two metals are bronze and invar. = ( 2 - 1 )L (d. T/dr) Q = 1. 06 o Physics 151: Lecture 37, Pg 14

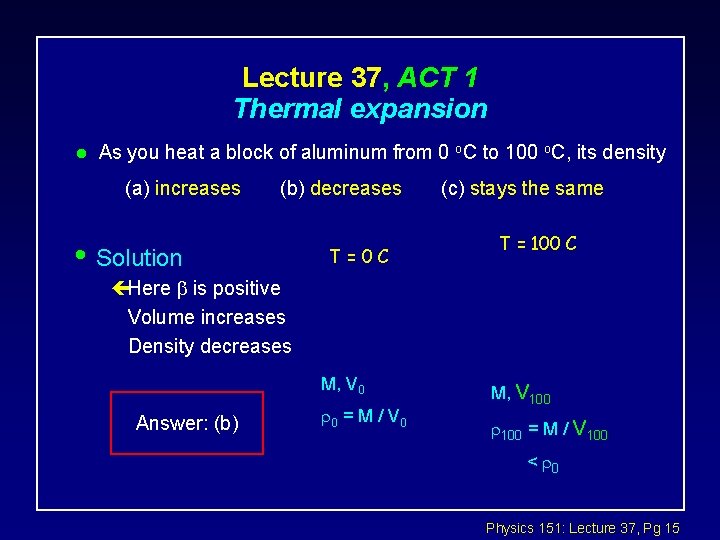
Lecture 37, ACT 1 Thermal expansion l As you heat a block of aluminum from 0 o. C to 100 o. C, its density (a) increases (b) decreases • Solution T=0 C (c) stays the same T = 100 C çHere is positive Volume increases Density decreases M, V 0 Answer: (b) 0 = M / V 0 M, V 100 = M / V 100 < 0 Physics 151: Lecture 37, Pg 15

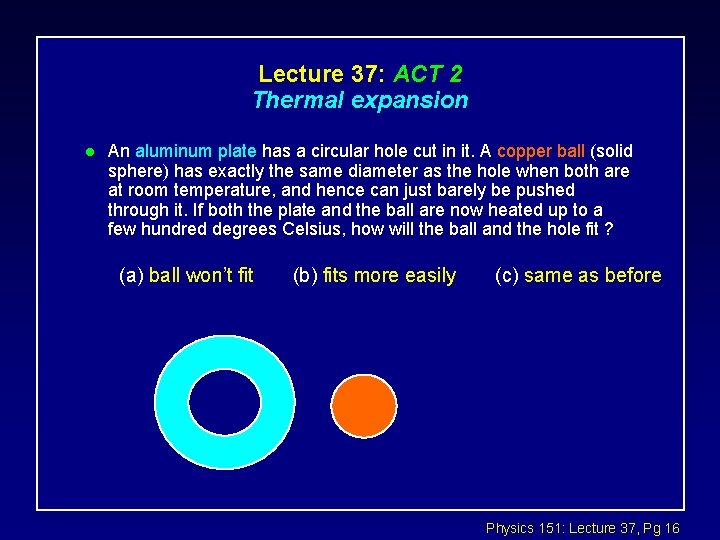
Lecture 37: ACT 2 Thermal expansion l An aluminum plate has a circular hole cut in it. A copper ball (solid sphere) has exactly the same diameter as the hole when both are at room temperature, and hence can just barely be pushed through it. If both the plate and the ball are now heated up to a few hundred degrees Celsius, how will the ball and the hole fit ? (a) ball won’t fit (b) fits more easily (c) same as before Physics 151: Lecture 37, Pg 16

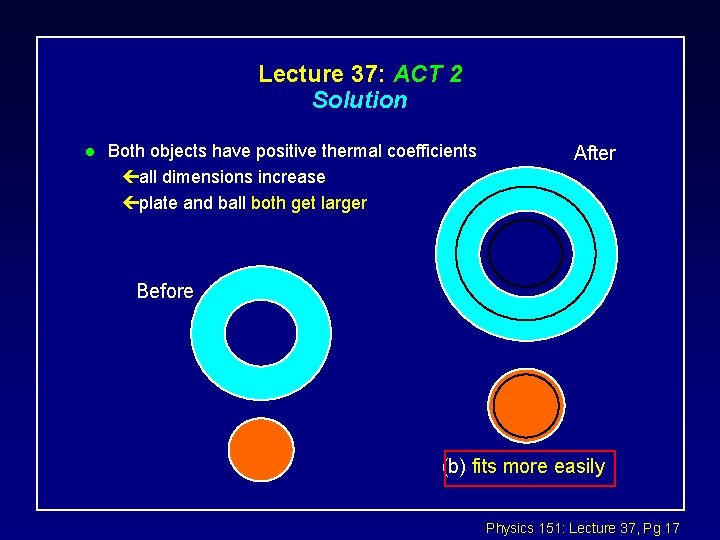
Lecture 37: ACT 2 Solution l Both objects have positive thermal coefficients çall dimensions increase çplate and ball both get larger After Before (b) fits more easily Physics 151: Lecture 37, Pg 17

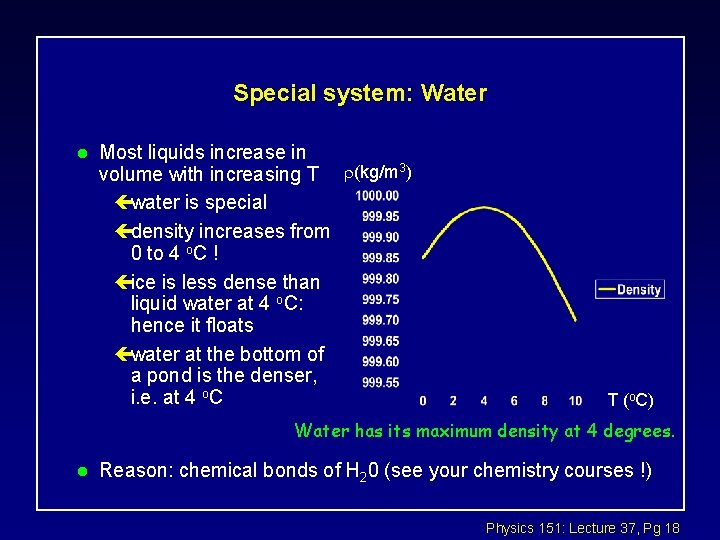
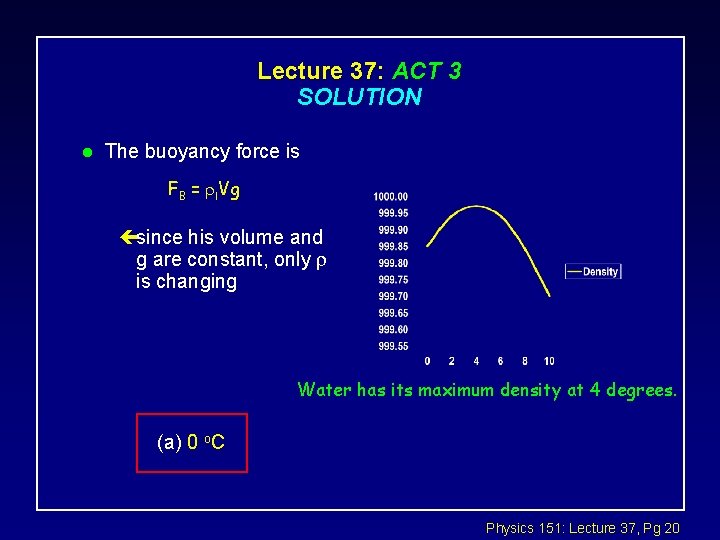
Special system: Water l Most liquids increase in volume with increasing T (kg/m 3) çwater is special çdensity increases from 0 to 4 o. C ! çice is less dense than liquid water at 4 o. C: hence it floats çwater at the bottom of a pond is the denser, i. e. at 4 o. C T (o. C) Water has its maximum density at 4 degrees. l Reason: chemical bonds of H 20 (see your chemistry courses !) Physics 151: Lecture 37, Pg 18

Lecture 37: ACT 3 l Not being a great athlete, and having lots of money to spend, Gill Bates decides to keep the lake in his back yard at the exact temperature which will maximize the buoyant force on him when he swims. Which of the following would be the best choice? (a) 0 o. C (b) 4 o. C (c) 32 o. C (d) 100 o. C (e) 212 o. C Physics 151: Lecture 37, Pg 19

Lecture 37: ACT 3 SOLUTION l The buoyancy force is FB = l. Vg çsince his volume and g are constant, only is changing Water has its maximum density at 4 degrees. (a) 0 o. C Physics 151: Lecture 37, Pg 20

Lecture 37: Problem 1 l A liquid with a coefficient of volume expansion just fills a spherical shell of volume Vi at a temperature of Ti (see Fig. to the right). The shell is made of a material that has an average coefficient of linear expansion . The liquid is free to expand into an open capillary of area A projecting from the top of the sphere. l When the temperature increases by T how high does the liquid rises in the capillary ( h ) ? h l =(Vi /A) ( - 3 ) T For a typical system, such as a mercury thermometer, why is it a good approximation to neglect the expansion of the shell ? (for glass) << (for mercury), so ( -3 ) within 5%. Physics 151: Lecture 37, Pg 21

Ideal gas l l Consider a gas in a container of volume V, at pressure P, and at temperature T Equation of state çLinks these quantities çGenerally very complicated: but not for ideal gas m=mass M=mass of one mole n = m/M : number of moles One mole contains NA=6. 022 X 1023 particles : Avogadro’s number • Equation of state for an ideal gas PV = n. RT R is called the universal gas constant In SI units, R =8. 315 J / mol·K Physics 151: Lecture 37, Pg 22

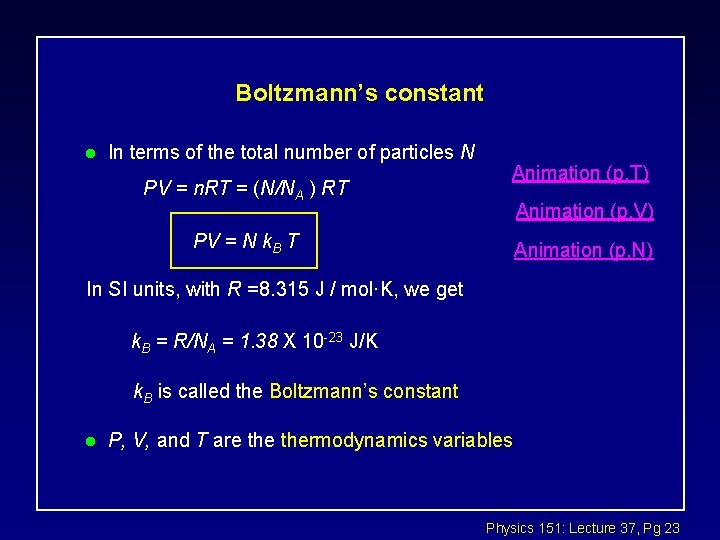
Boltzmann’s constant l In terms of the total number of particles N PV = n. RT = (N/NA ) RT Animation (p, T) Animation (p, V) PV = N k. B T Animation (p, N) In SI units, with R =8. 315 J / mol·K, we get k. B = R/NA = 1. 38 X 10 -23 J/K k. B is called the Boltzmann’s constant l P, V, and T are thermodynamics variables Physics 151: Lecture 37, Pg 23

l l Lecture 37: Problem 2 A vertical cylinder of crosssectional area A=0. 001 m 2 is fitted with a tight-fitting, frictionless piston of mass m = 20. 0 kg (see the Fig. To the right) If n = 0. 200 moles of an ideal gas are in the cylinder at a temperature of T = 350 K, what is the height h at which the piston is in equilibrium under its own weight ? h = n R T/(mg + PA) h = 1. 94 m Physics 151: Lecture 37, Pg 24

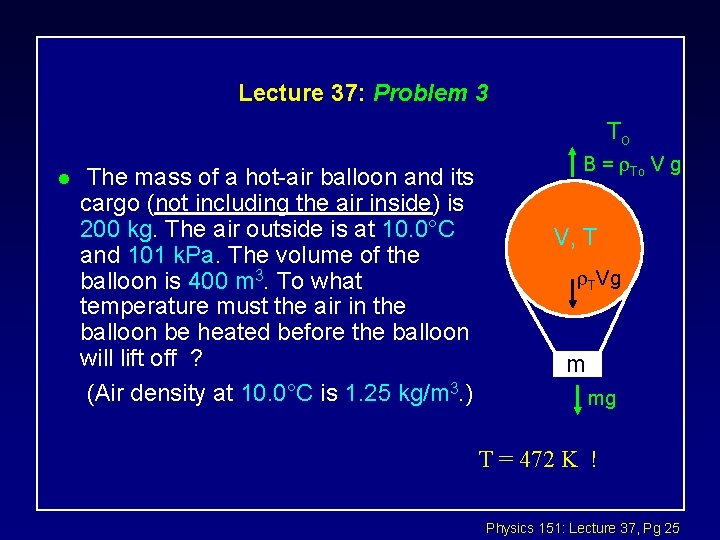
Lecture 37: Problem 3 To l The mass of a hot-air balloon and its cargo (not including the air inside) is 200 kg. The air outside is at 10. 0°C and 101 k. Pa. The volume of the balloon is 400 m 3. To what temperature must the air in the balloon be heated before the balloon will lift off ? (Air density at 10. 0°C is 1. 25 kg/m 3. ) B = To V g V, T TVg m mg T = 472 K ! Physics 151: Lecture 37, Pg 25

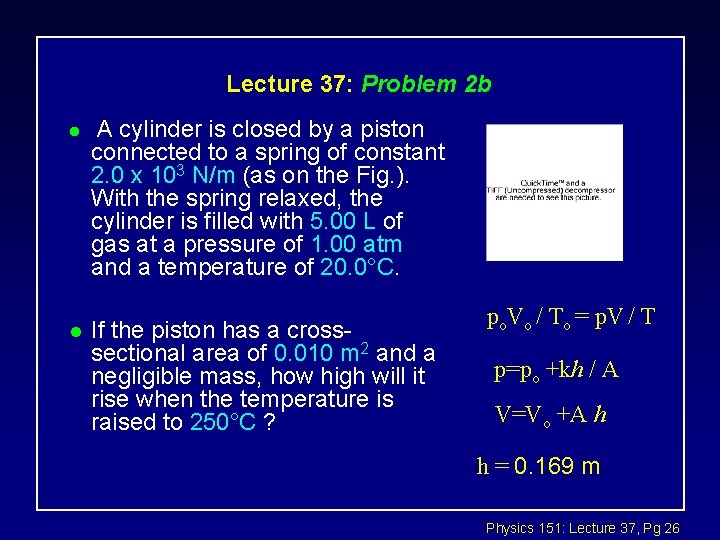
Lecture 37: Problem 2 b l l A cylinder is closed by a piston connected to a spring of constant 2. 0 x 103 N/m (as on the Fig. ). With the spring relaxed, the cylinder is filled with 5. 00 L of gas at a pressure of 1. 00 atm and a temperature of 20. 0°C. If the piston has a crosssectional area of 0. 010 m 2 and a negligible mass, how high will it rise when the temperature is raised to 250°C ? po. Vo / To = p. V / T p=po +kh / A V=Vo +A h h = 0. 169 m Physics 151: Lecture 37, Pg 26

Recap of today’s lecture l Chap. 19: temperature çTemperature and Zeroth Law of Thermodynamics çTemperature scales çThermal expansion çIdeal gas Physics 151: Lecture 37, Pg 27
 Todays agenda
Todays agenda Hr meeting agenda topics
Hr meeting agenda topics 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Agenda sistemica y agenda institucional
Agenda sistemica y agenda institucional Ib physics ia ideas mechanics
Ib physics ia ideas mechanics Action research topics in physics
Action research topics in physics Nuclear physics topics for presentation
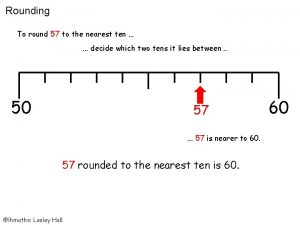
Nuclear physics topics for presentation 51 rounded to the nearest ten
51 rounded to the nearest ten Chm 151 final exam
Chm 151 final exam Model netics
Model netics Conforme a tua infinita graça numero
Conforme a tua infinita graça numero Econ 151
Econ 151 System software consists of
System software consists of 118/151
118/151 Cs 175 uci
Cs 175 uci Tổng kết vốn từ 151
Tổng kết vốn từ 151 Item 151
Item 151 Apsc 151
Apsc 151 1,151,725 bytes
1,151,725 bytes Freeze concentration
Freeze concentration Mcb 101 uiuc
Mcb 101 uiuc Econ 151
Econ 151 Scia antincendio esempio
Scia antincendio esempio Econ 151
Econ 151 Sjsu cs 151
Sjsu cs 151 Cs 151 sjsu
Cs 151 sjsu Physics 111 lecture notes
Physics 111 lecture notes Physics 101 lecture
Physics 101 lecture