PHYS 420 SPRING 2006 Dennis Papadopoulos LECTURE 10



- Slides: 37

PHYS 420 -SPRING 2006 Dennis Papadopoulos LECTURE 10 LIGHT II http: //physics. berea. edu/~king/Teaching/Mod. Phys/QM/Blackbody/Black. Body. html http: //physics. berea. edu/~king/Teaching/Mod. Phys/QM/Photoelectric. html http: //www. student. nada. kth. se/~f 93 -jhu/phys_sim/compton/Compton. htm

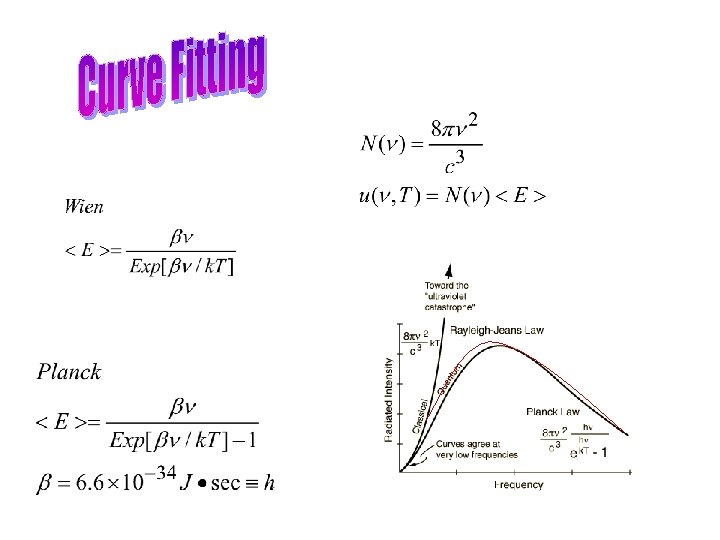
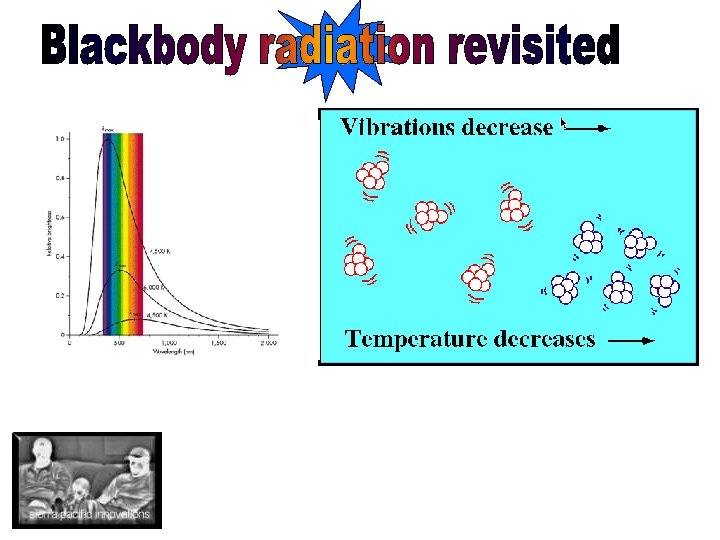
• Hertz showed that light is an electromagnetic phenomenon, and that electromagnetic waves behave much like any other wave-- they can be reflected, refracted, diffracted and polarized. • Heated solids emit a continuous spectrum of radiation whose intensity and spectral form depends only on their temperature (blackbody radiation) • Planck realized that the emission is caused by electrons oscillating on the surface of the body and emitting radiation att the frequency of their oscillation (little antennas) • However, unless energy is quantized, the radiation of a blackbody will continue to increase with frequency—a dilemma dubbed the ultraviolet catastrophe—forcing Planck to theorize that light comes in lumps and “oscillators” atomic walls must have quantized energy.

Black Body Radiation

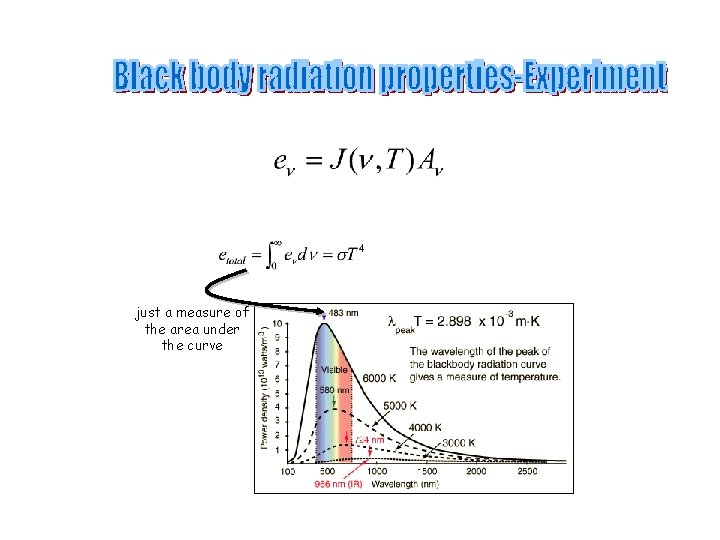
just a measure of the area under the curve

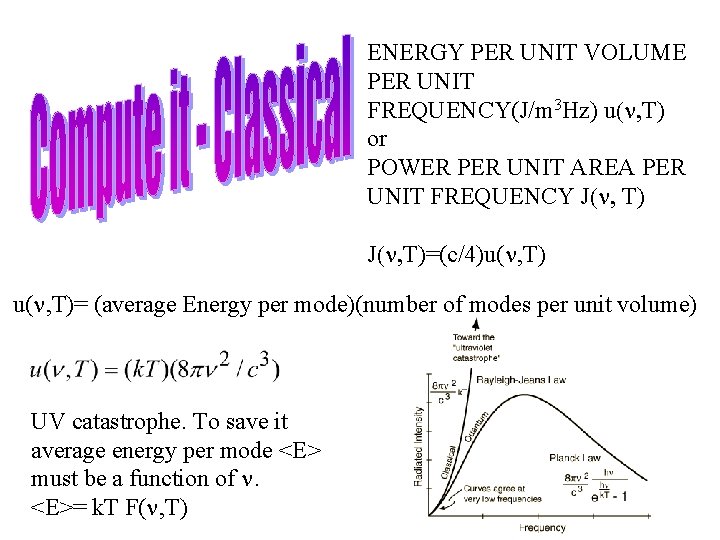
ENERGY PER UNIT VOLUME PER UNIT FREQUENCY(J/m 3 Hz) u(n, T) or POWER PER UNIT AREA PER UNIT FREQUENCY J(n, T)=(c/4)u(n, T)= (average Energy per mode)(number of modes per unit volume) UV catastrophe. To save it average energy per mode <E> must be a function of n. <E>= k. T F(n, T)


Particles have identical physical properties…but can be distinguished by following their (well defined) classical paths. See Ch. 10, section 10. 1 ASSUMPTIONS In equilibrium, the energy distribution of the particles will converge to the most probable allowed. In principle, there is no limit on the number of particles occupying each state.

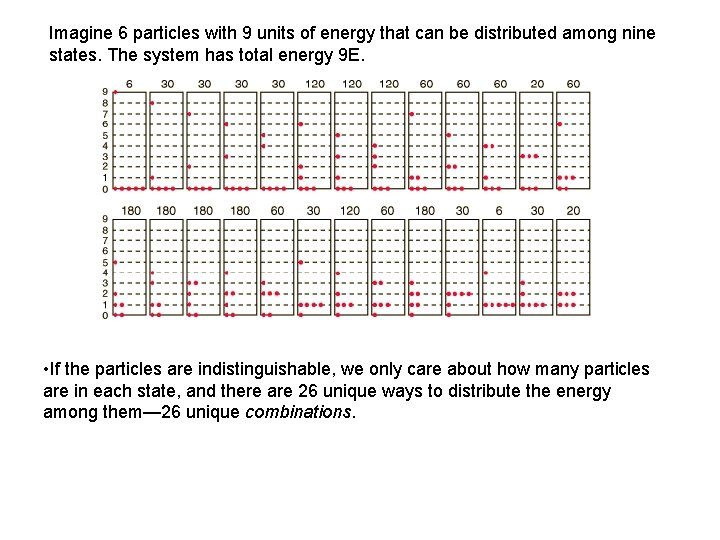
Imagine 6 particles with 9 units of energy that can be distributed among nine states. The system has total energy 9 E. • If the particles are indistinguishable, we only care about how many particles are in each state, and there are 26 unique ways to distribute the energy among them— 26 unique combinations.

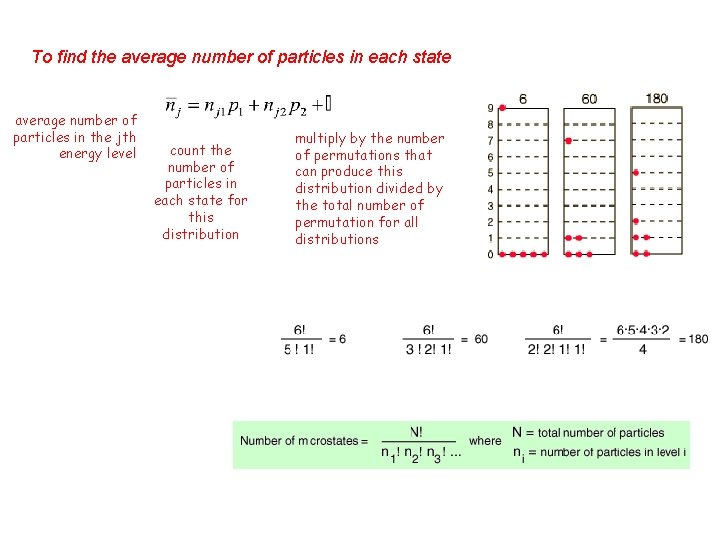
To find the average number of particles in each state average number of particles in the jth energy level count the number of particles in each state for this distribution multiply by the number of permutations that can produce this distribution divided by the total number of permutation for all distributions

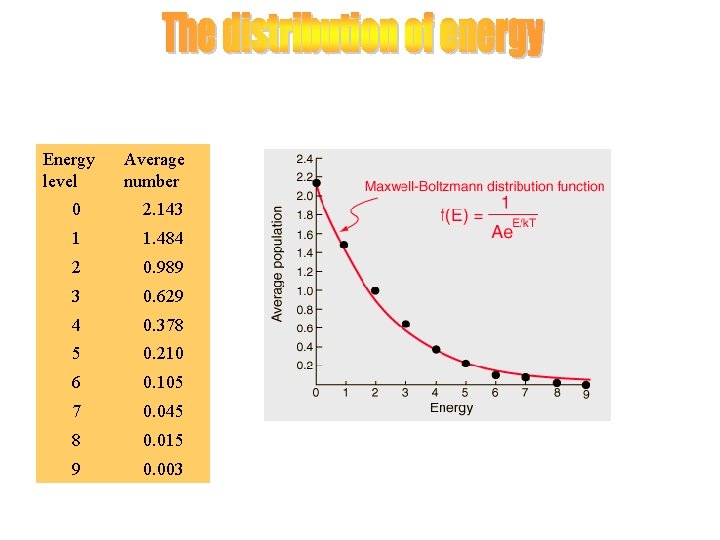
Energy level Average number 0 2. 143 1 1. 484 2 0. 989 3 0. 629 4 0. 378 5 0. 210 6 0. 105 7 0. 045 8 0. 015 9 0. 003

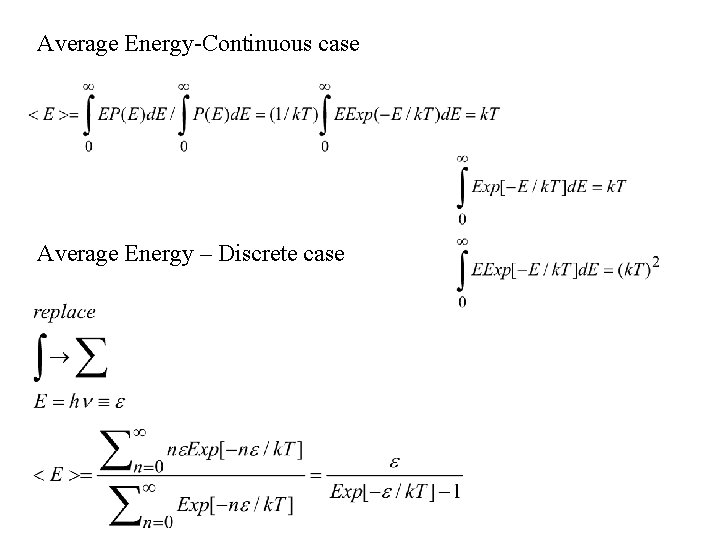
Average Energy-Continuous case Average Energy – Discrete case

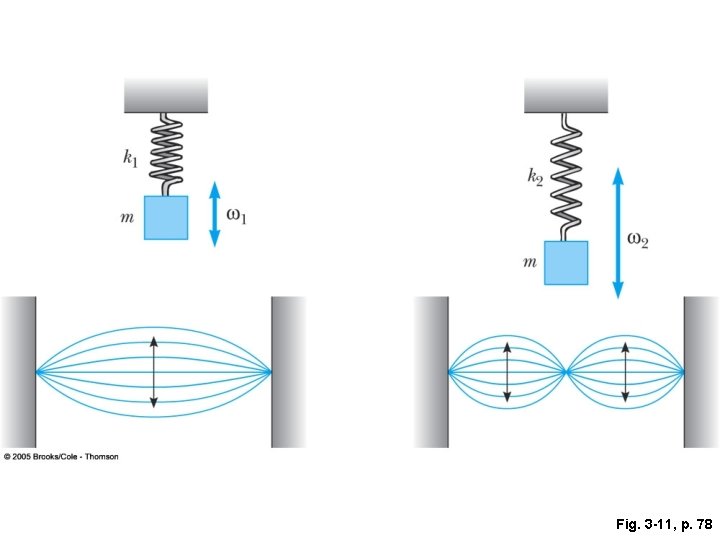
Fig. 3 -11, p. 78

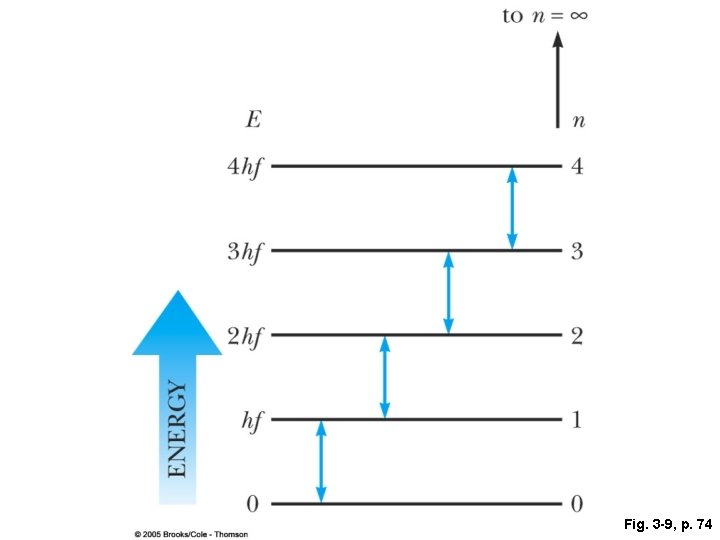
Fig. 3 -9, p. 74

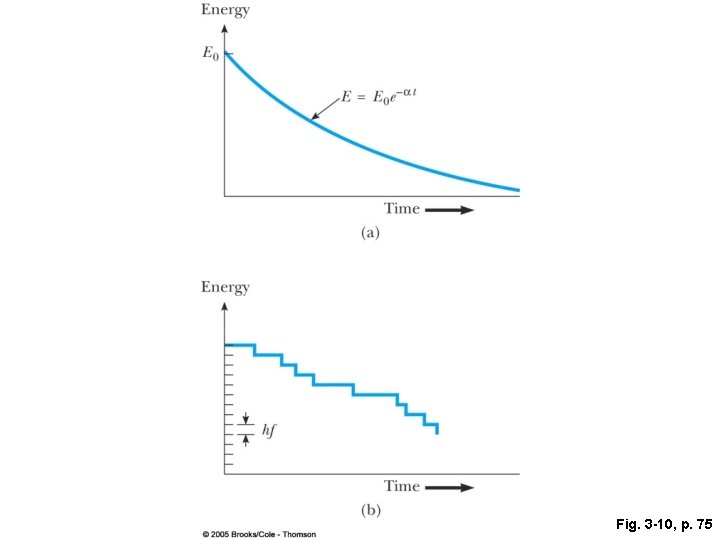
Fig. 3 -10, p. 75

• Inconsistency in Planck’s thinking in that he • Quantized the oscillators emitting radiation in the walls of the cavity • Insisted that the cavity radiation was composed of classical waves • Concluded that radiation must be composed of lumps ( quantas) consstent with the quantas emitted by the cavity walls

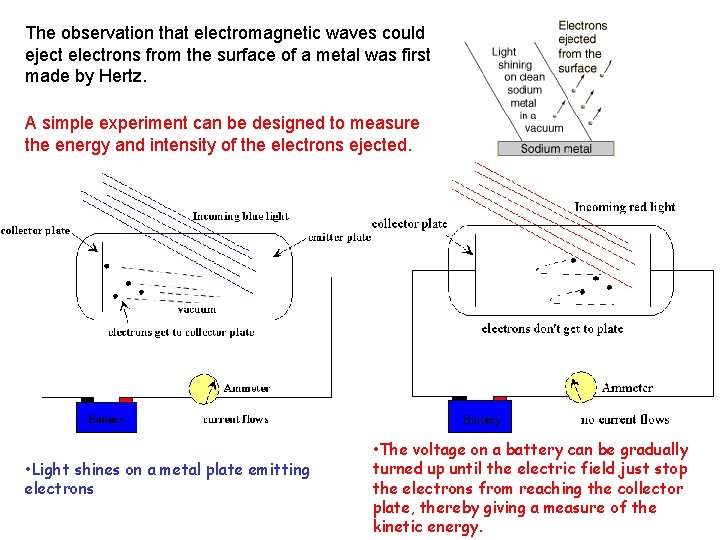
The observation that electromagnetic waves could eject electrons from the surface of a metal was first made by Hertz. A simple experiment can be designed to measure the energy and intensity of the electrons ejected. • Light shines on a metal plate emitting electrons • The voltage on a battery can be gradually turned up until the electric field just stop the electrons from reaching the collector plate, thereby giving a measure of the kinetic energy.

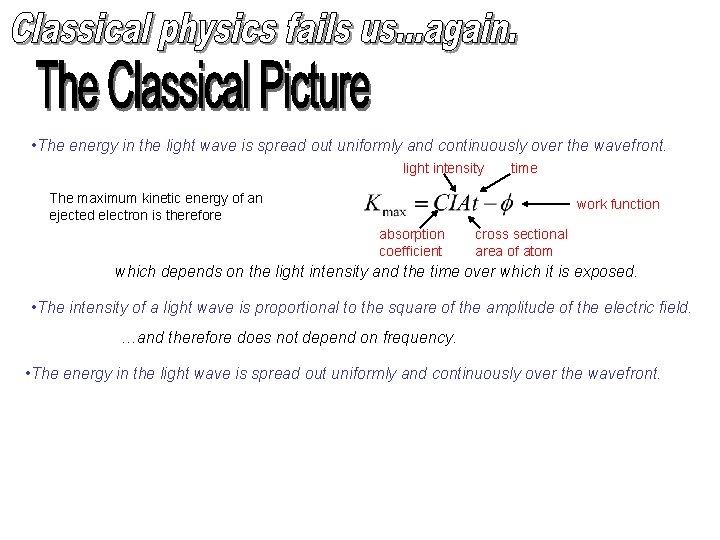
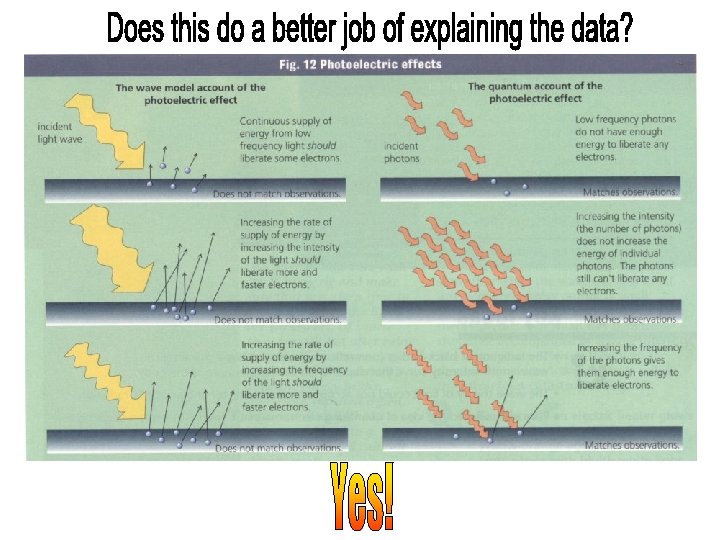
• The energy in the light wave is spread out uniformly and continuously over the wavefront. light intensity time The maximum kinetic energy of an ejected electron is therefore work function absorption coefficient cross sectional area of atom which depends on the light intensity and the time over which it is exposed. • The intensity of a light wave is proportional to the square of the amplitude of the electric field. …and therefore does not depend on frequency. • The energy in the light wave is spread out uniformly and continuously over the wavefront.

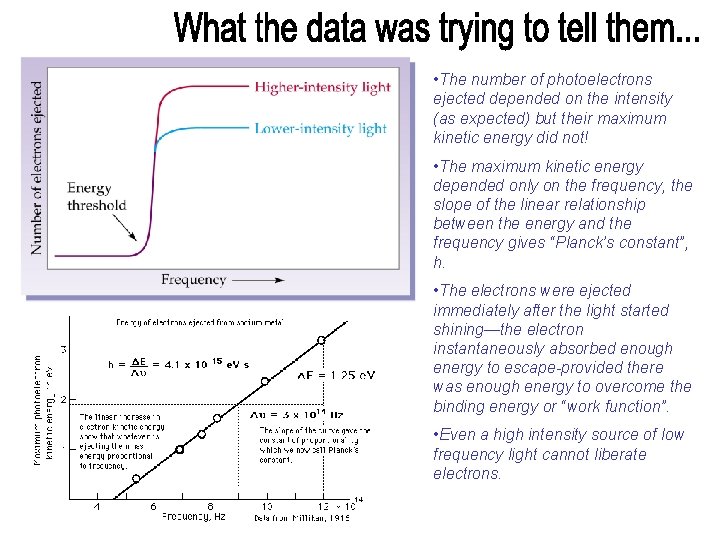
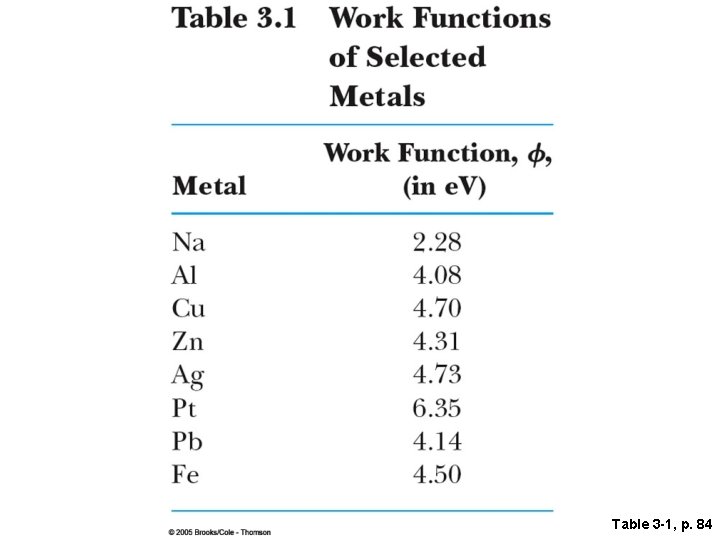
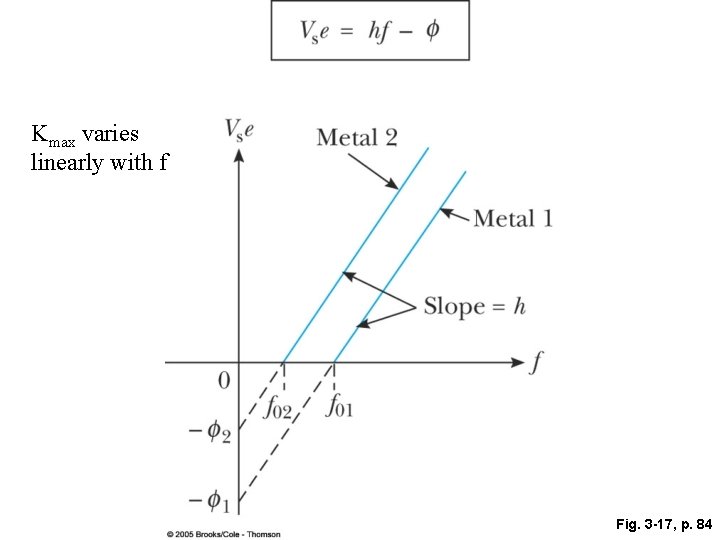
• The number of photoelectrons ejected depended on the intensity (as expected) but their maximum kinetic energy did not! • The maximum kinetic energy depended only on the frequency, the slope of the linear relationship between the energy and the frequency gives “Planck’s constant”, h. • The electrons were ejected immediately after the light started shining—the electron instantaneously absorbed enough energy to escape-provided there was enough energy to overcome the binding energy or “work function”. • Even a high intensity source of low frequency light cannot liberate electrons.

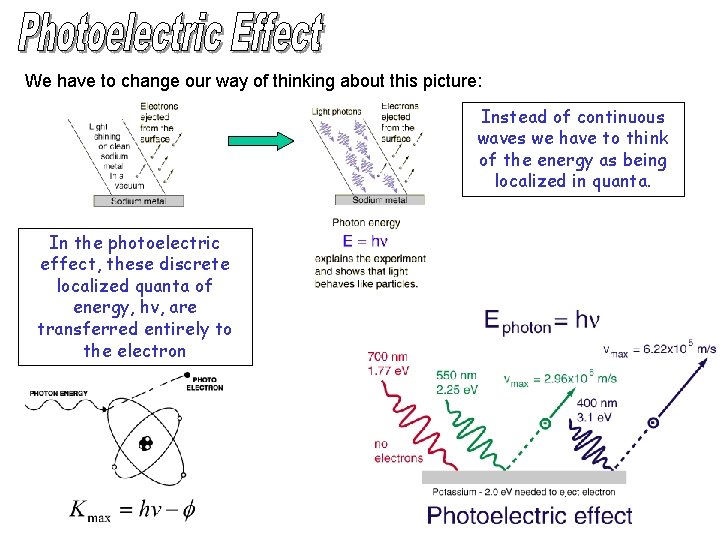
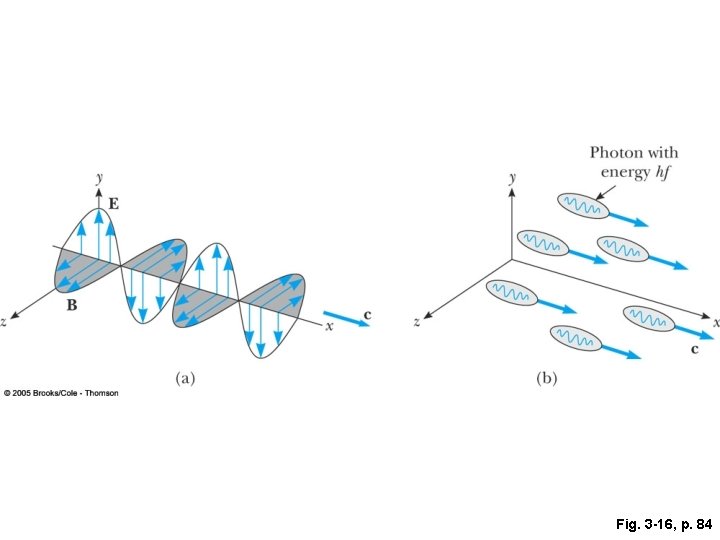
We have to change our way of thinking about this picture: Instead of continuous waves we have to think of the energy as being localized in quanta. In the photoelectric effect, these discrete localized quanta of energy, hv, are transferred entirely to the electron


Fig. 3 -16, p. 84

Table 3 -1, p. 84

Kmax varies linearly with f Fig. 3 -17, p. 84

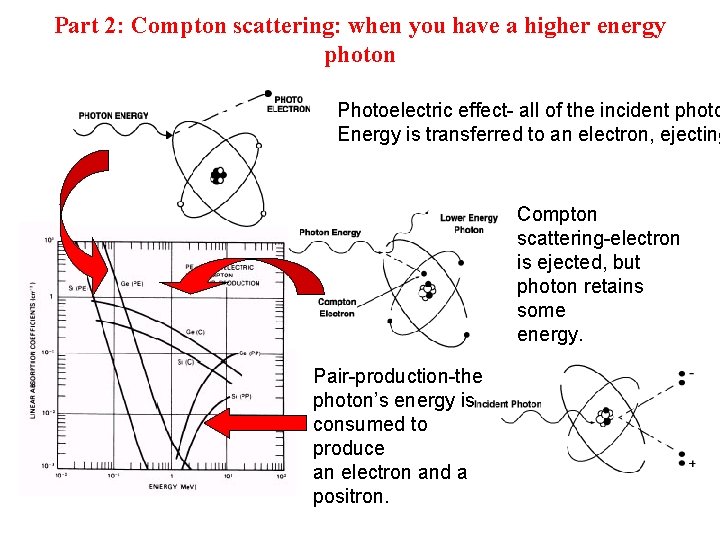
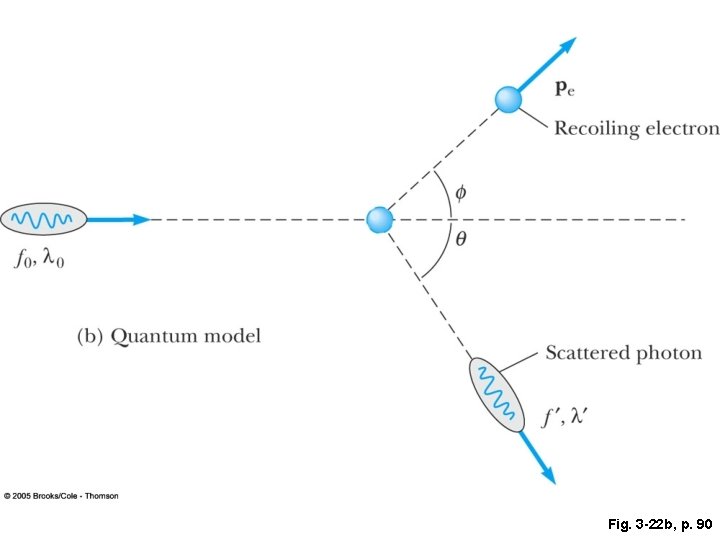
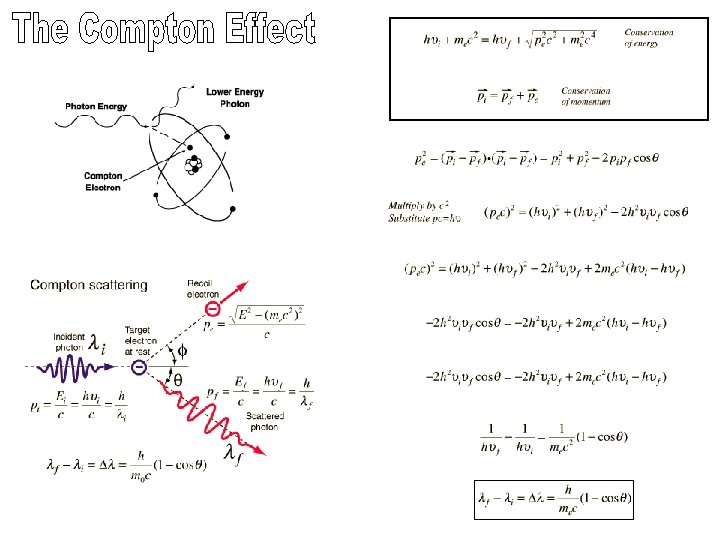
Part 2: Compton scattering: when you have a higher energy photon Photoelectric effect- all of the incident photo Energy is transferred to an electron, ejecting Compton scattering-electron is ejected, but photon retains some energy. Pair-production-the photon’s energy is consumed to produce an electron and a positron.

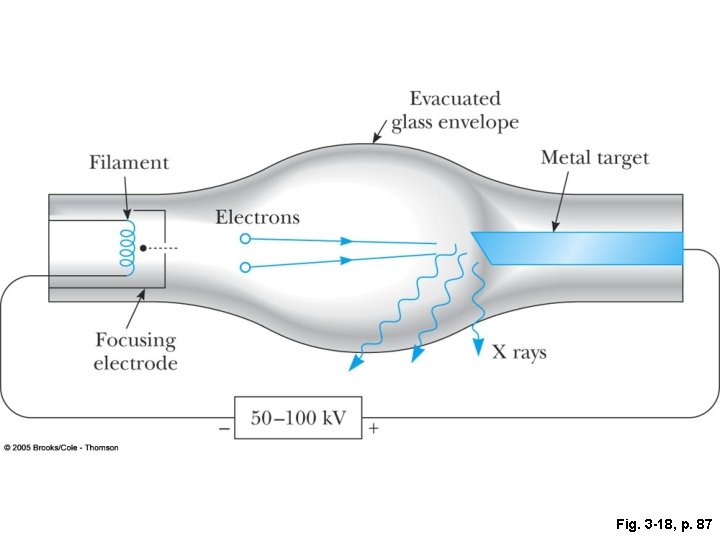
Fig. 3 -18, p. 87

Fig. 3 -19, p. 87

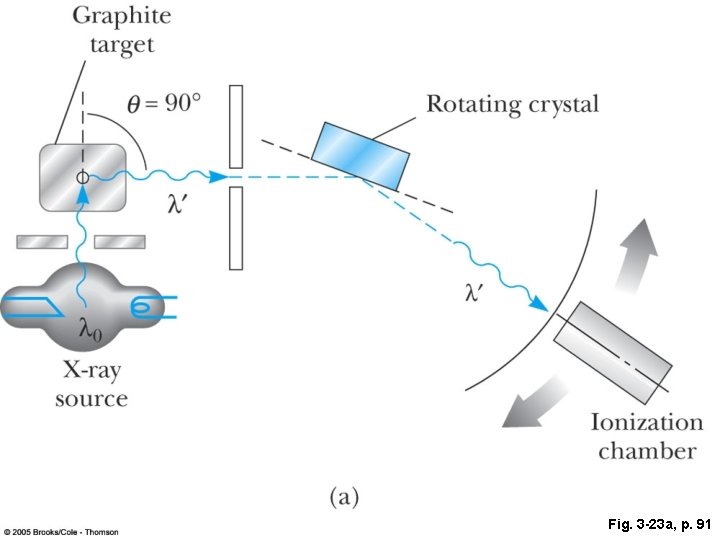
Fig. 3 -23 a, p. 91

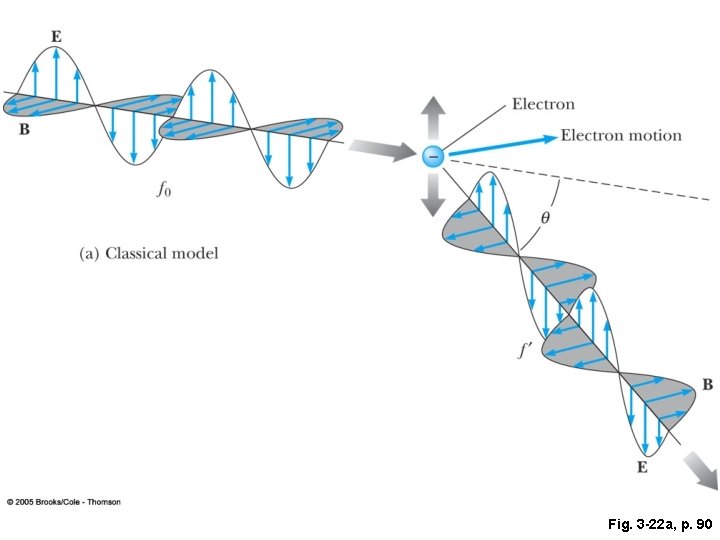
Fig. 3 -22 a, p. 90

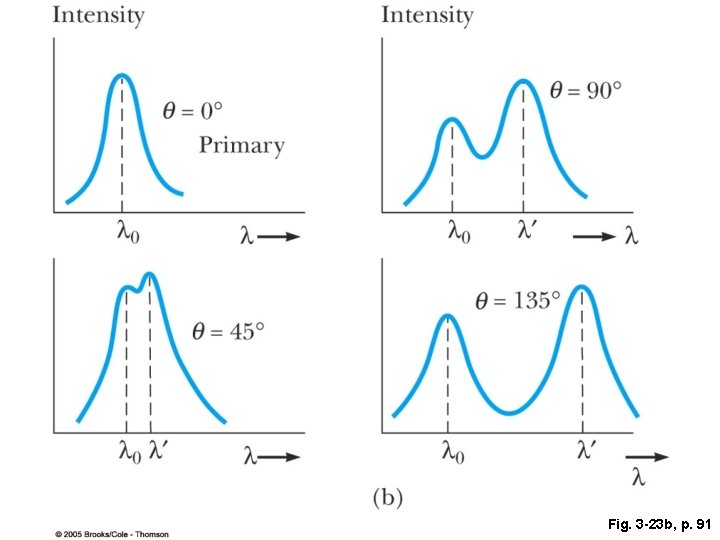
Fig. 3 -23 b, p. 91

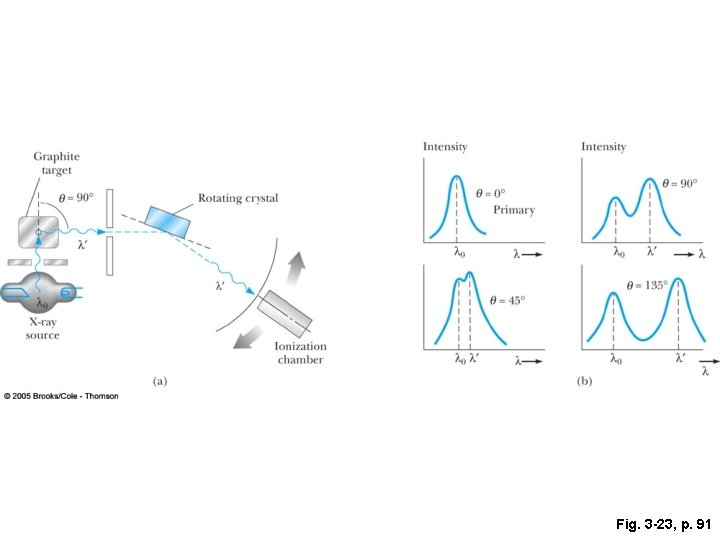
Fig. 3 -23, p. 91

Fig. 3 -22 b, p. 90


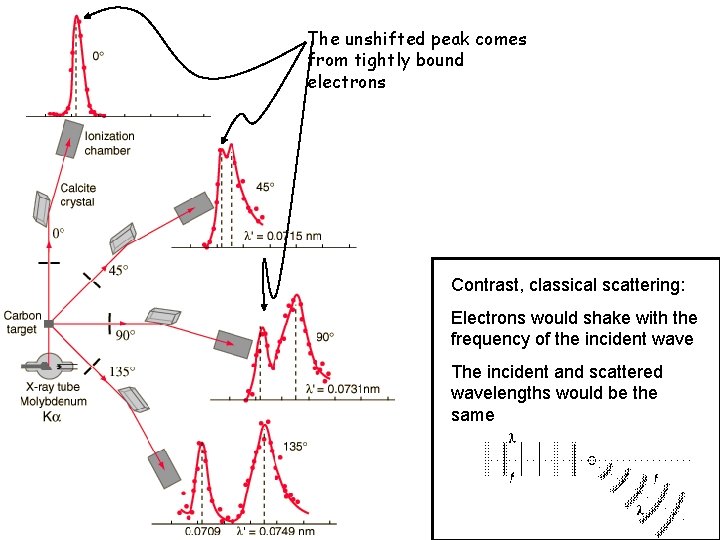
The unshifted peak comes from tightly bound electrons Contrast, classical scattering: Electrons would shake with the frequency of the incident wave The incident and scattered wavelengths would be the same

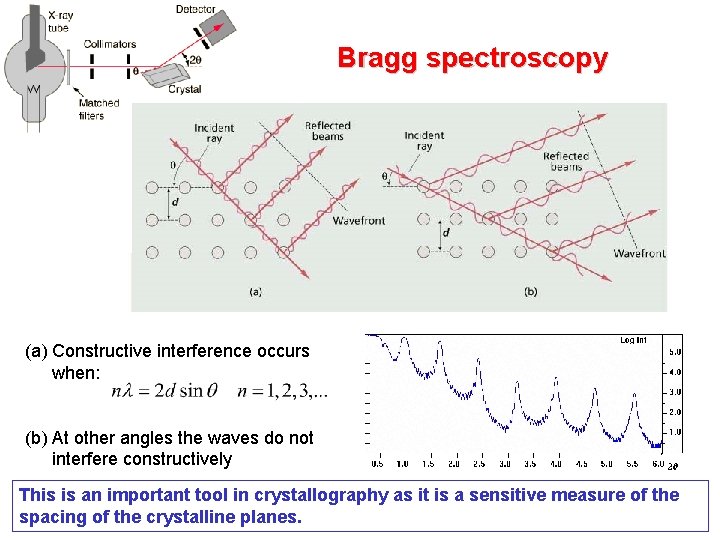
Bragg spectroscopy (a) Constructive interference occurs when: (b) At other angles the waves do not interfere constructively This is an important tool in crystallography as it is a sensitive measure of the spacing of the crystalline planes.


Light: • Interferes like a wave • Scatters like a particle • Diffracts like a wave • Can be polarized like a wave …and the answer is…drumroll please… If light, which was previously thought of as a wave, has characteristics of particles, could it be true that particles must also be thought of as waves in some contexts in order to fully describe their behavior?

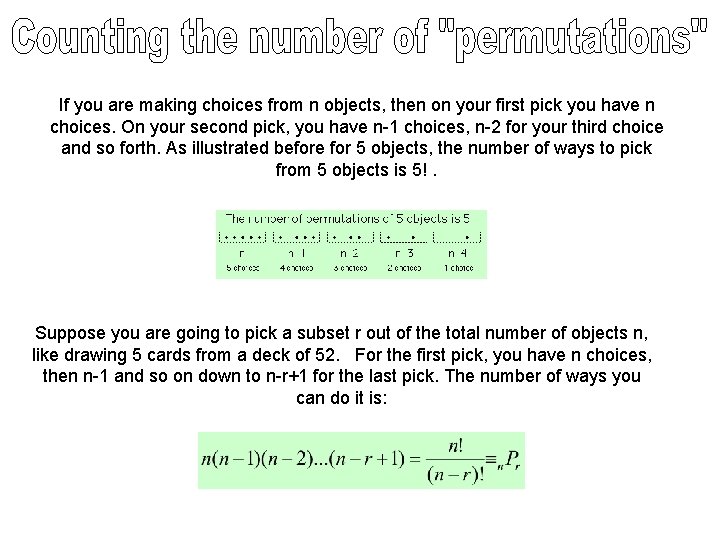
If you are making choices from n objects, then on your first pick you have n choices. On your second pick, you have n-1 choices, n-2 for your third choice and so forth. As illustrated before for 5 objects, the number of ways to pick from 5 objects is 5!. Suppose you are going to pick a subset r out of the total number of objects n, like drawing 5 cards from a deck of 52. For the first pick, you have n choices, then n-1 and so on down to n-r+1 for the last pick. The number of ways you can do it is: