PHYS 420 SPRING 2006 Dennis Papadopoulos LECTURE 9






- Slides: 26

PHYS 420 -SPRING 2006 Dennis Papadopoulos LECTURE 9 LIGHT I

What is the nature of Part I

• Hertz shows that light is an electromagnetic phenomenon, and that electromagnetic waves behave much like any other wave-- they can be reflected, refracted, diffracted and polarized. • Heated solids emit a continuous spectrum of radiation whose intensity and spectral form depends only on their temperature (blackbody radiation) • However, unless energy is quantized, the radiation of a blackbody will continue to increase with frequency—a dilemma dubbed the ultraviolet catastrophe—forcing Planck to theorize that light comes in lumps and “oscillators” atomic walls must have quantized energy.

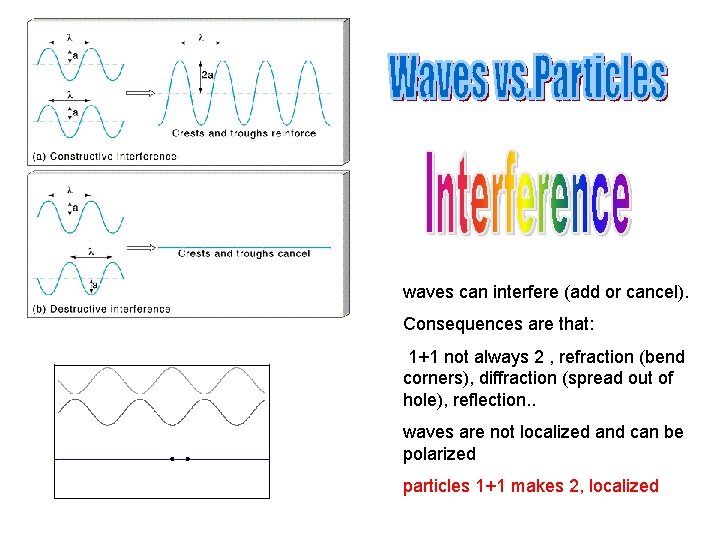
waves can interfere (add or cancel). Consequences are that: 1+1 not always 2 , refraction (bend corners), diffraction (spread out of hole), reflection. . waves are not localized and can be polarized particles 1+1 makes 2, localized

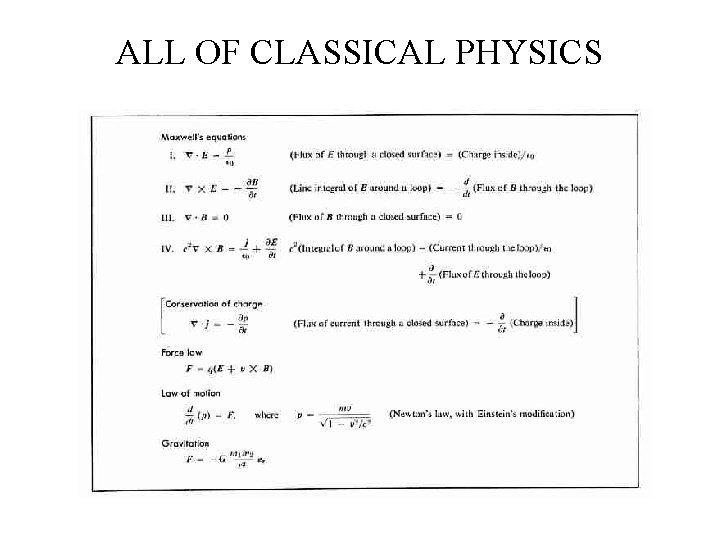
ALL OF CLASSICAL PHYSICS

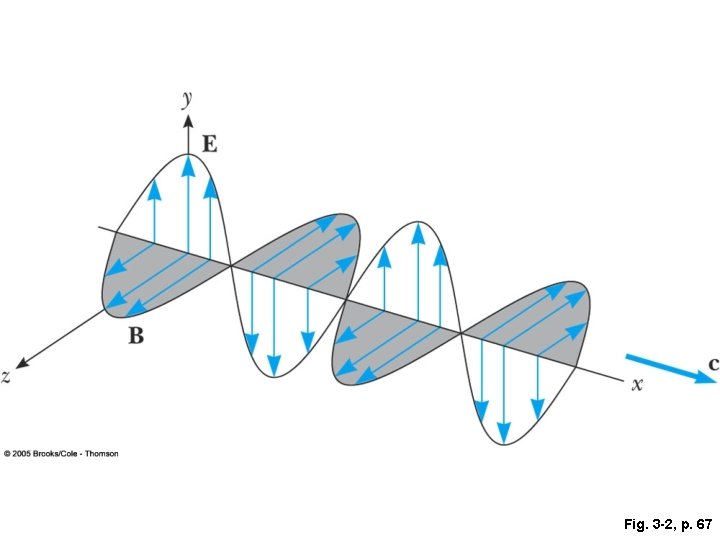
Fig. 3 -2, p. 67



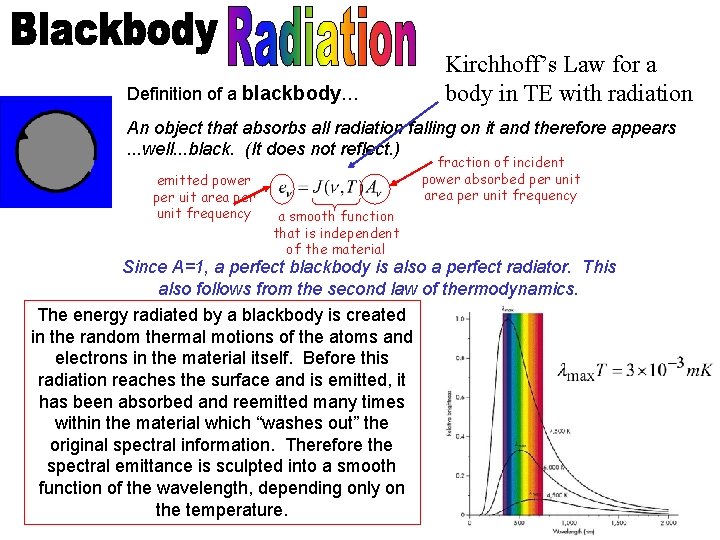
The invention of radio? Hertz proves that light is really an electromagnetic wave. Waves could be generated in one circuit, and electric pulses with the same frequency could be induced in an antenna some distance away. These electromagnetic waves could be reflected, and refracted, focused, polarized, and made to interfere—just like light!

The mystery: In 1792, the famous china-maker, Thomas Wedgewood, noticed that all hot objects became red at the same temperature independent of size, composition, etc… Now, for a bit background of thermodynamics…. You may have noticed yourself that things glow (i. e. emit light) when they heat up. your stove… Unlike gases, solids do not have characteristic lines. a glassblower blowing glass… the sun and other stars. Teaser: Why these characteristic lines? You’ll find out shortly.

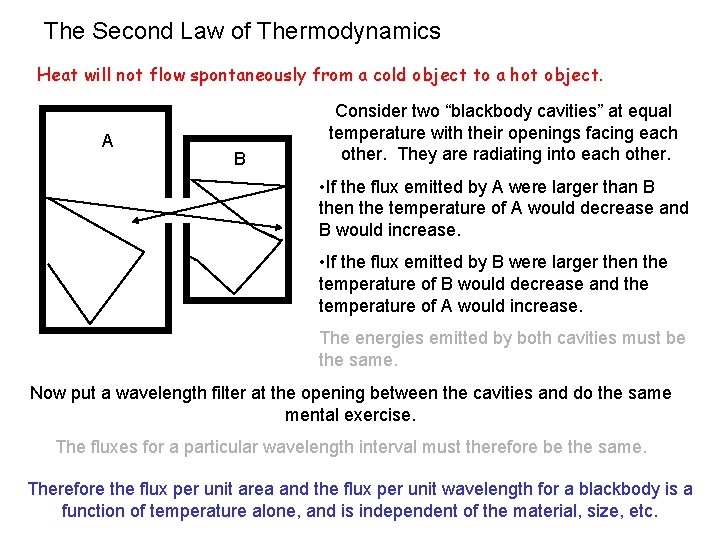
Definition of a blackbody… Kirchhoff’s Law for a body in TE with radiation An object that absorbs all radiation falling on it and therefore appears. . . well. . . black. (It does not reflect. ) emitted power per uit area per unit frequency fraction of incident power absorbed per unit area per unit frequency a smooth function that is independent of the material Since A=1, a perfect blackbody is also a perfect radiator. This also follows from the second law of thermodynamics. The energy radiated by a blackbody is created in the random thermal motions of the atoms and electrons in the material itself. Before this radiation reaches the surface and is emitted, it has been absorbed and reemitted many times within the material which “washes out” the original spectral information. Therefore the spectral emittance is sculpted into a smooth function of the wavelength, depending only on the temperature.

The Second Law of Thermodynamics Heat will not flow spontaneously from a cold object to a hot object. A B Consider two “blackbody cavities” at equal temperature with their openings facing each other. They are radiating into each other. • If the flux emitted by A were larger than B then the temperature of A would decrease and B would increase. • If the flux emitted by B were larger then the temperature of B would decrease and the temperature of A would increase. The energies emitted by both cavities must be the same. Now put a wavelength filter at the opening between the cavities and do the same mental exercise. The fluxes for a particular wavelength interval must therefore be the same. Therefore the flux per unit area and the flux per unit wavelength for a blackbody is a function of temperature alone, and is independent of the material, size, etc.

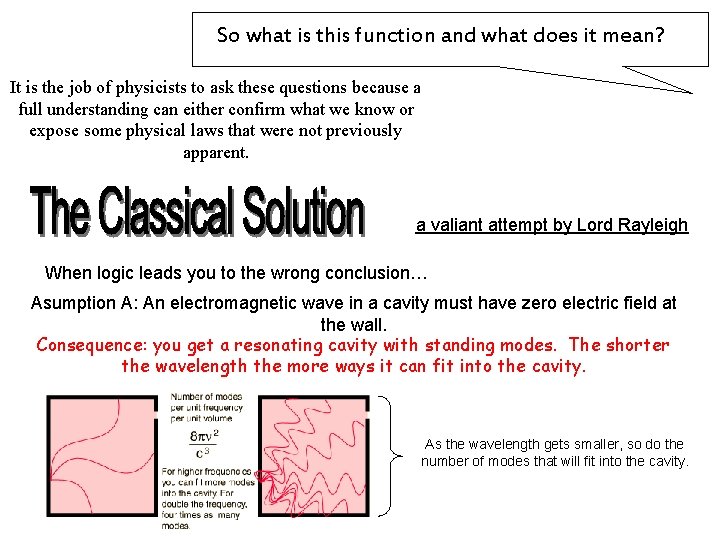
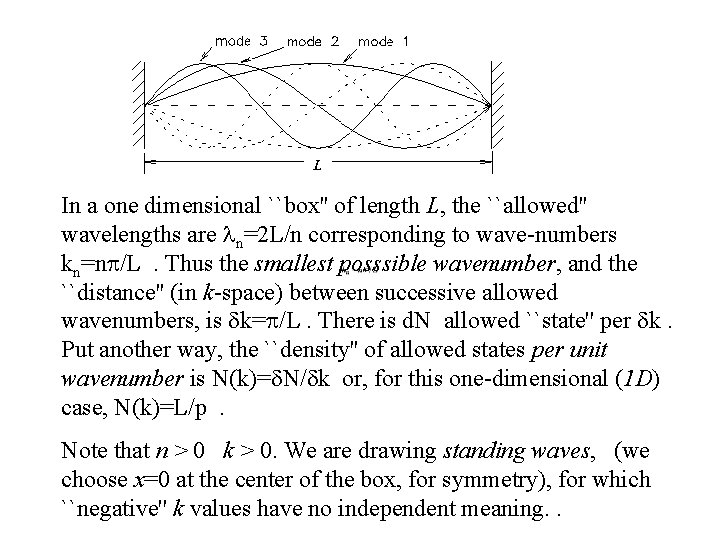
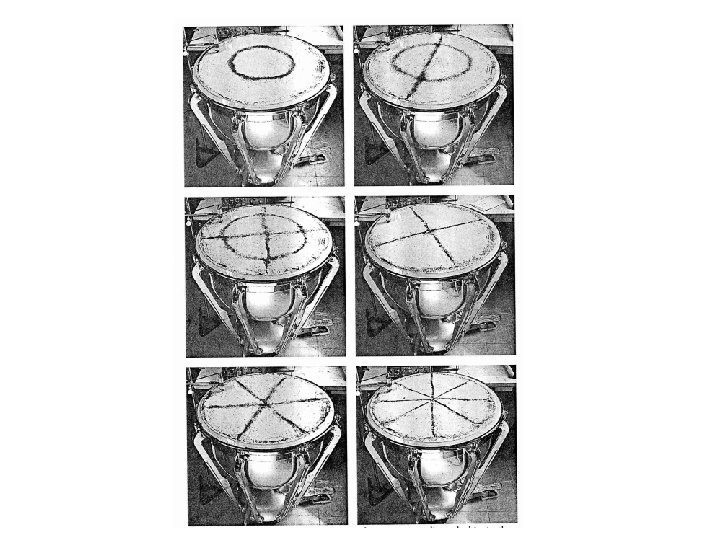
So what is this function and what does it mean? It is the job of physicists to ask these questions because a full understanding can either confirm what we know or expose some physical laws that were not previously apparent. a valiant attempt by Lord Rayleigh When logic leads you to the wrong conclusion… Asumption A: An electromagnetic wave in a cavity must have zero electric field at the wall. Consequence: you get a resonating cavity with standing modes. The shorter the wavelength the more ways it can fit into the cavity. As the wavelength gets smaller, so do the number of modes that will fit into the cavity.

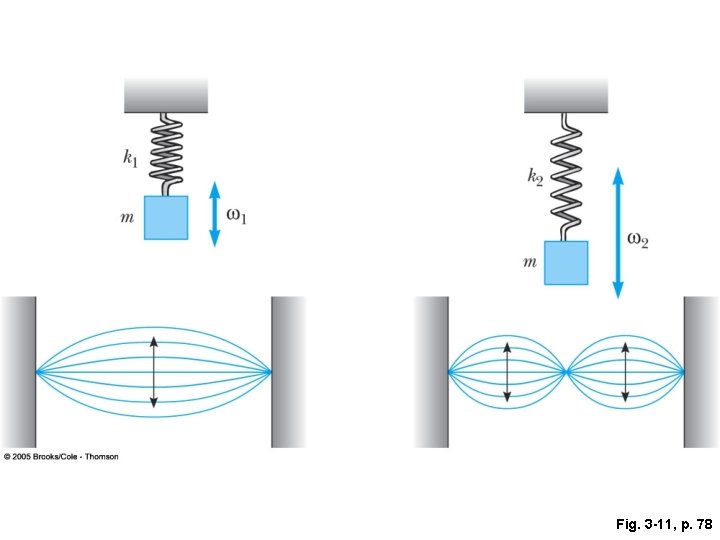
Fig. 3 -11, p. 78

In a one dimensional ``box'' of length L, the ``allowed'' wavelengths are ln=2 L/n corresponding to wave-numbers kn=np/L. Thus the smallest posssible wavenumber, and the ``distance'' (in k-space) between successive allowed wavenumbers, is dk=p/L. There is d. N allowed ``state'' per dk. Put another way, the ``density'' of allowed states per unit wavenumber is N(k)=d. N/dk or, for this one-dimensional (1 D) case, N(k)=L/p. Note that n > 0 k > 0. We are drawing standing waves, (we choose x=0 at the center of the box, for symmetry), for which ``negative'' k values have no independent meaning. .





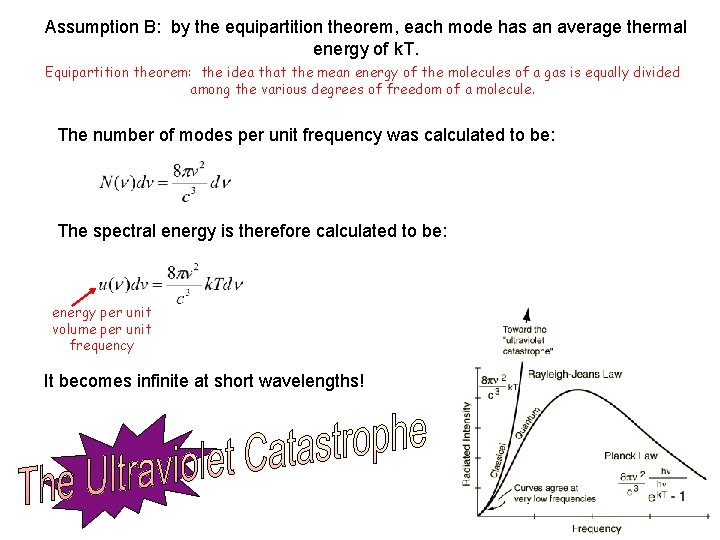
Assumption B: by the equipartition theorem, each mode has an average thermal energy of k. T. Equipartition theorem: the idea that the mean energy of the molecules of a gas is equally divided among the various degrees of freedom of a molecule. The number of modes per unit frequency was calculated to be: The spectral energy is therefore calculated to be: energy per unit volume per unit frequency It becomes infinite at short wavelengths!

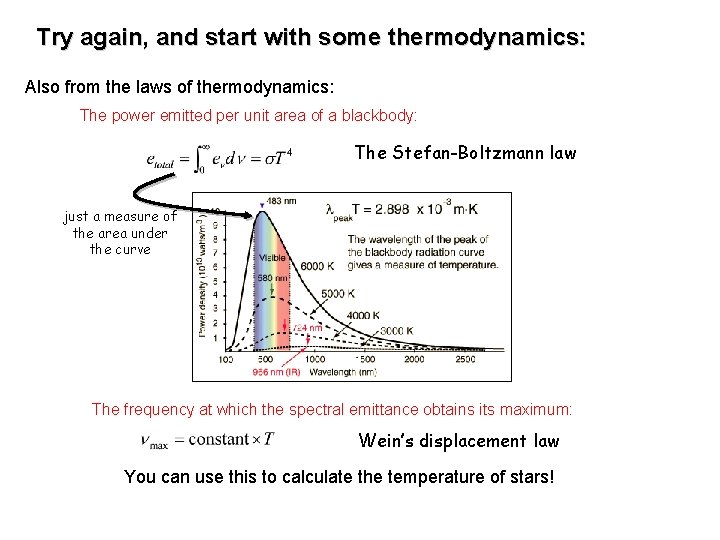
Try again, and start with some thermodynamics: Also from the laws of thermodynamics: The power emitted per unit area of a blackbody: The Stefan-Boltzmann law just a measure of the area under the curve The frequency at which the spectral emittance obtains its maximum: Wein’s displacement law You can use this to calculate the temperature of stars!

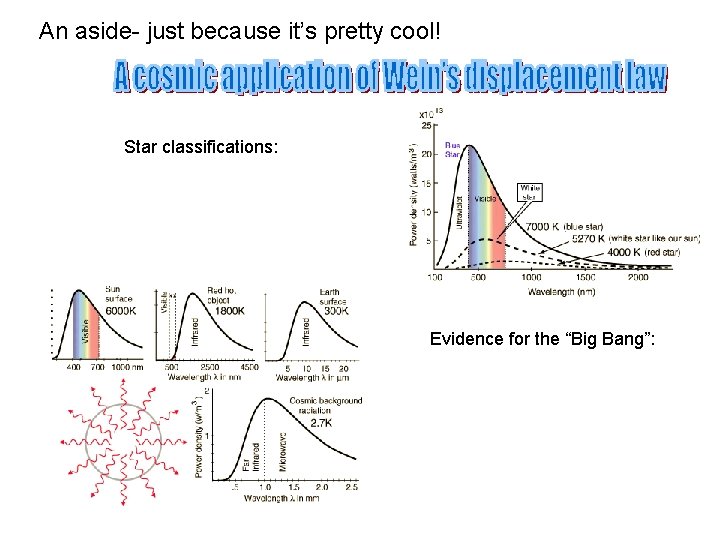
An aside- just because it’s pretty cool! Star classifications: Evidence for the “Big Bang”:

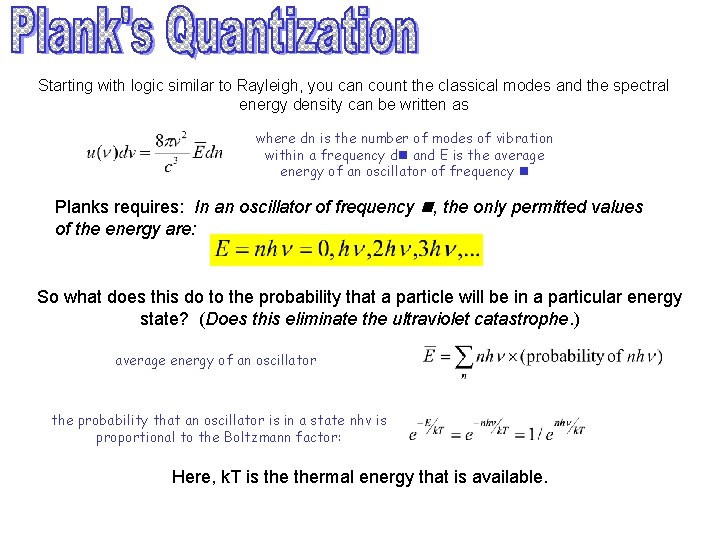
Starting with logic similar to Rayleigh, you can count the classical modes and the spectral energy density can be written as where dn is the number of modes of vibration within a frequency dn and E is the average energy of an oscillator of frequency n Planks requires: In an oscillator of frequency n, the only permitted values of the energy are: So what does this do to the probability that a particle will be in a particular energy state? (Does this eliminate the ultraviolet catastrophe. ) average energy of an oscillator the probability that an oscillator is in a state nhv is proportional to the Boltzmann factor: Here, k. T is thermal energy that is available.

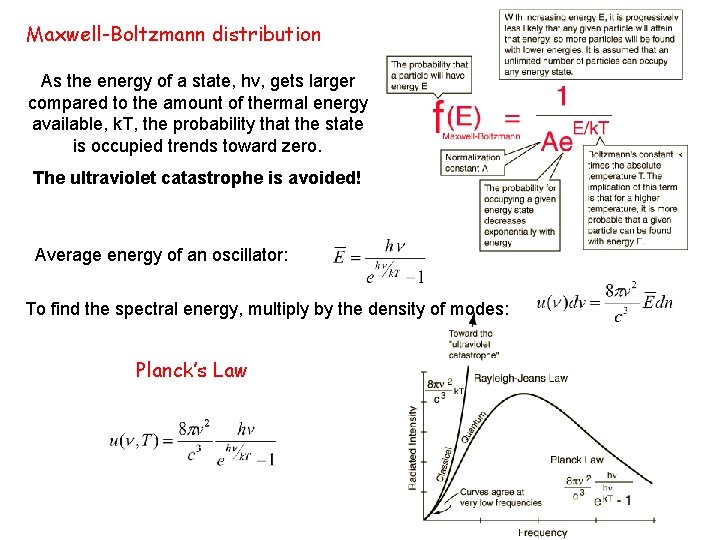
Maxwell-Boltzmann distribution As the energy of a state, hv, gets larger compared to the amount of thermal energy available, k. T, the probability that the state is occupied trends toward zero. The ultraviolet catastrophe is avoided! Average energy of an oscillator: To find the spectral energy, multiply by the density of modes: Planck’s Law

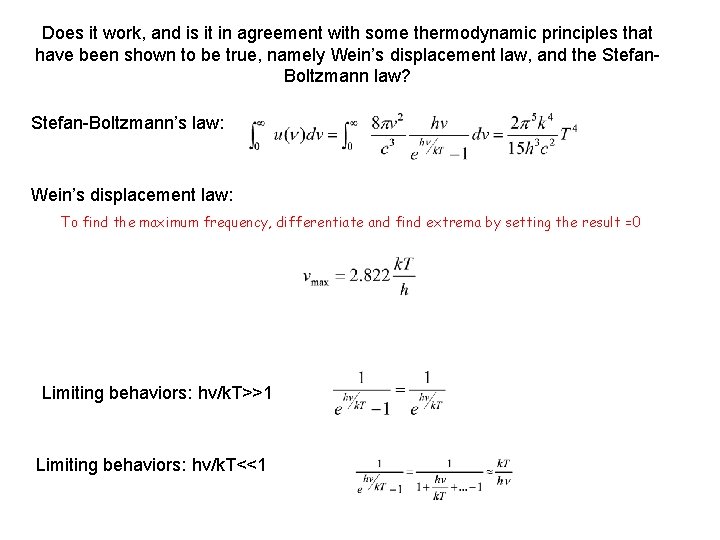
Does it work, and is it in agreement with some thermodynamic principles that have been shown to be true, namely Wein’s displacement law, and the Stefan. Boltzmann law? Stefan-Boltzmann’s law: Wein’s displacement law: To find the maximum frequency, differentiate and find extrema by setting the result =0 Limiting behaviors: hv/k. T>>1 Limiting behaviors: hv/k. T<<1

So now we’ve found evidence of the quantization of energy. What does that say about the nature of light? Tune in next time… Credit: Many of the figures in this lecture came from Kara Hoffman’s website, from http: //hyperphysics. phy-astr. gsu and from Richard Feynmann Physics Lectures