PHENOLIC SECRETS Exploring Finishing Tannins in Maturation and









- Slides: 20

PHENOLIC SECRETS: Exploring Finishing Tannins in Maturation and Blending Time: 9: 45 am – 10: 45 am Moderator: Tinus Els Speakers: Adolfo Alarcon – Winemaker / Technical Liason, Trinchero Family Wine Estates Sabrina Lueck – Instructor of Enology, Walla Community College Richard Mansfield – North American Winemaker, Winery Exchange Bernard Pradel – Co-Founder, Toasted Oak Co.

Finishing Tannins: Function, Fates, and Flavors Alternate Title: “Sabrina reads literature so you don’t have to!” Sabrina Bitz Lueck Walla Community College / College Cellars

Today • • • Difference between fermentation, ageing, and finishing tannins Difference between hydrolysable and condensed tannins Different hydrolysable tannin species • What do they do? Different oak aroma species • What do they do? Compare 4 finishing tannin products using ETS Laboratories data

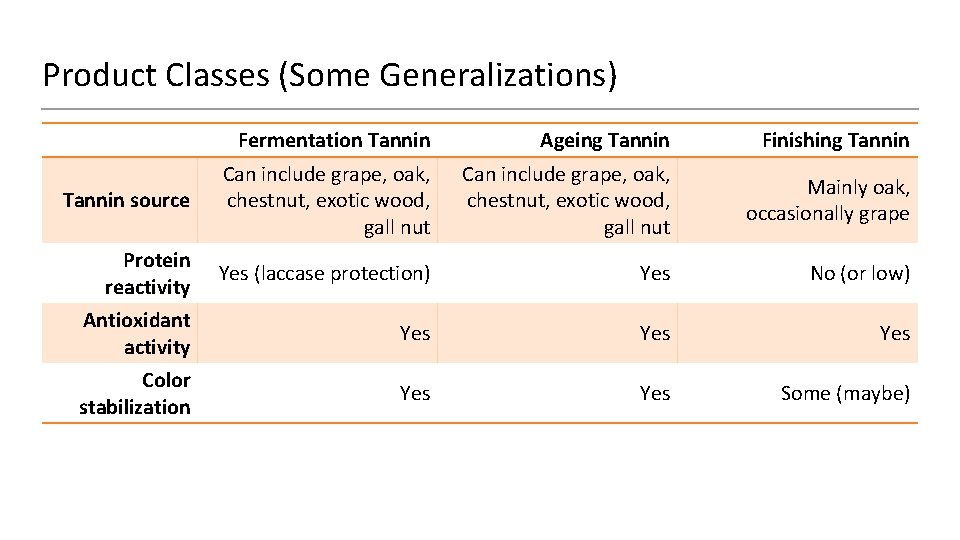
Product Classes (Some Generalizations) Fermentation Tannin Ageing Tannin Finishing Tannin source Can include grape, oak, chestnut, exotic wood, gall nut Mainly oak, occasionally grape Protein reactivity Yes (laccase protection) Yes No (or low) Antioxidant activity Yes Yes Color stabilization Yes Some (maybe)

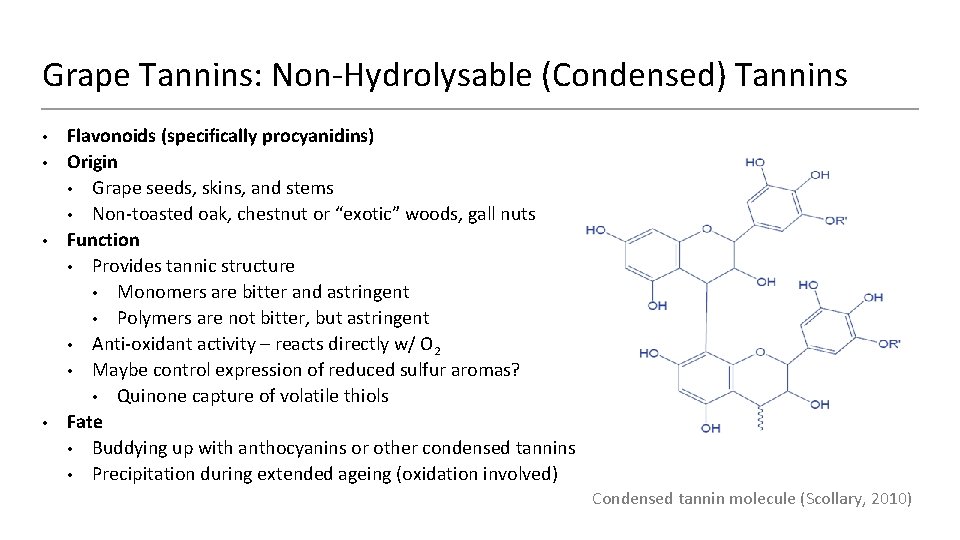
Grape Tannins: Non-Hydrolysable (Condensed) Tannins • • Flavonoids (specifically procyanidins) Origin • Grape seeds, skins, and stems • Non-toasted oak, chestnut or “exotic” woods, gall nuts Function • Provides tannic structure • Monomers are bitter and astringent • Polymers are not bitter, but astringent • Anti-oxidant activity – reacts directly w/ O 2 • Maybe control expression of reduced sulfur aromas? • Quinone capture of volatile thiols Fate • Buddying up with anthocyanins or other condensed tannins • Precipitation during extended ageing (oxidation involved) Condensed tannin molecule (Scollary, 2010)

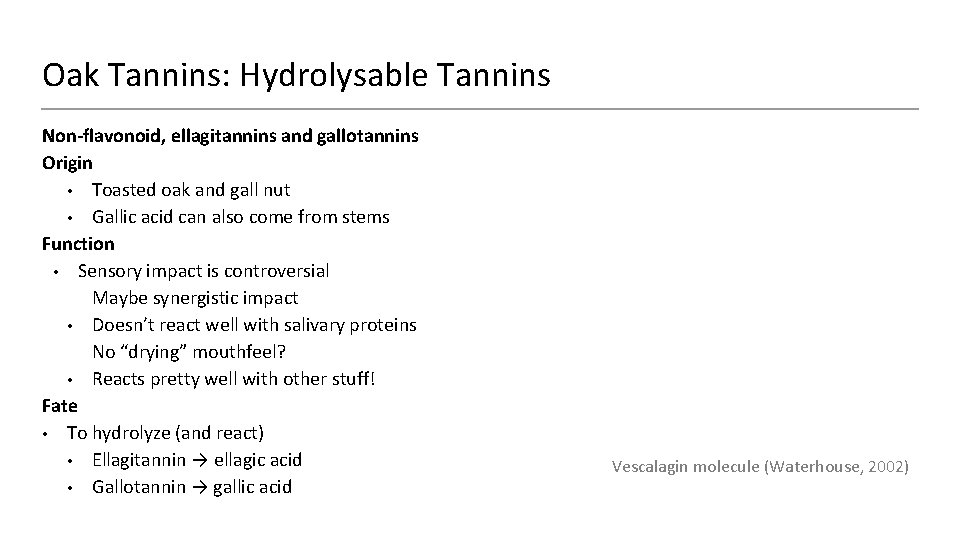
Oak Tannins: Hydrolysable Tannins Non-flavonoid, ellagitannins and gallotannins Origin • Toasted oak and gall nut • Gallic acid can also come from stems Function • Sensory impact is controversial Maybe synergistic impact • Doesn’t react well with salivary proteins No “drying” mouthfeel? • Reacts pretty well with other stuff! Fate • To hydrolyze (and react) • Ellagitannin → ellagic acid • Gallotannin → gallic acid Vescalagin molecule (Waterhouse, 2002)

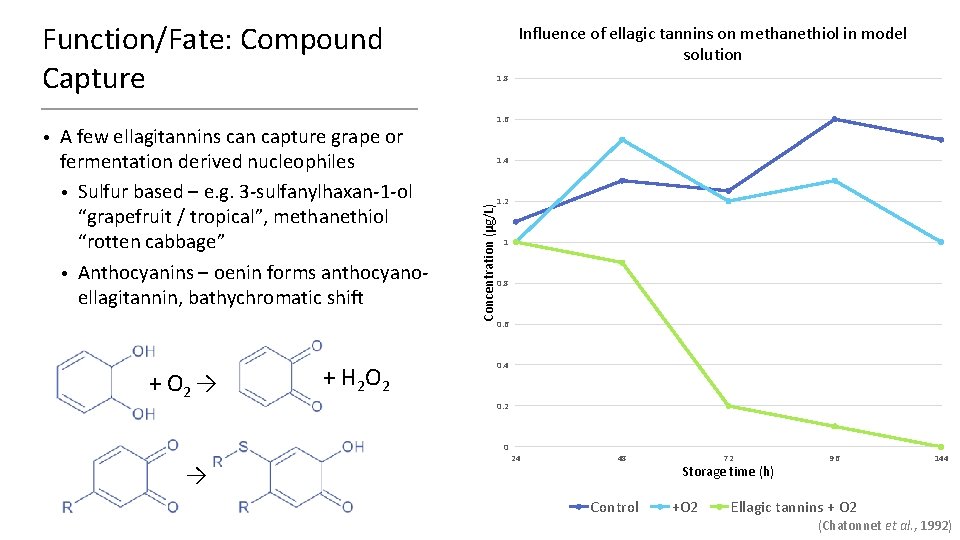
Function/Fate: Compound Capture A few ellagitannins can capture grape or fermentation derived nucleophiles • Sulfur based – e. g. 3 -sulfanylhaxan-1 -ol “grapefruit / tropical”, methanethiol “rotten cabbage” • Anthocyanins – oenin forms anthocyanoellagitannin, bathychromatic shift + O 2 → + H 2 O 2 1. 8 1. 6 1. 4 Concentration (μg/L) • Influence of ellagic tannins on methanethiol in model solution 1. 2 1 0. 8 0. 6 0. 4 0. 2 0 → 24 48 Control 72 Storage time (h) +O 2 96 144 Ellagic tannins + O 2 (Chatonnet et al. , 1992)

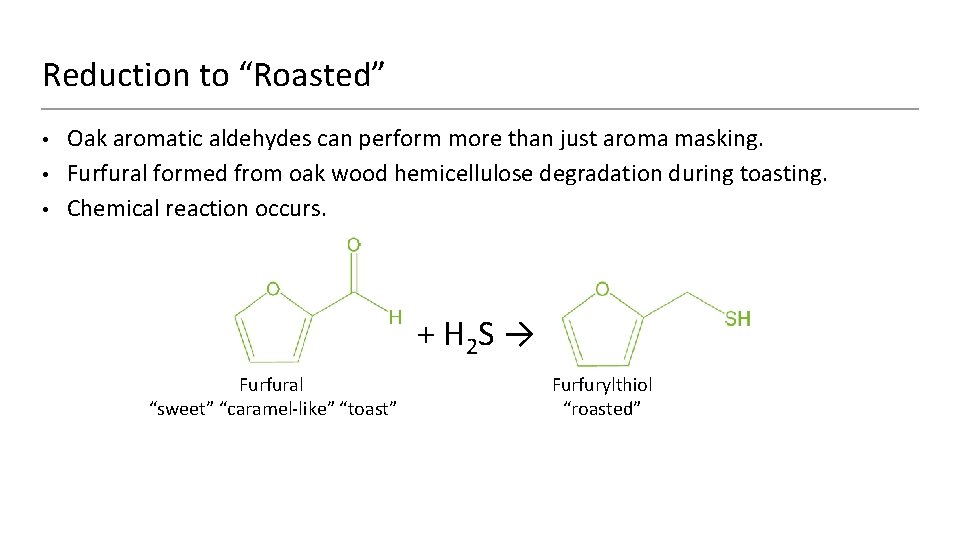
Reduction to “Roasted” • • • Oak aromatic aldehydes can perform more than just aroma masking. Furfural formed from oak wood hemicellulose degradation during toasting. Chemical reaction occurs. + H 2 S → Furfural “sweet” “caramel-like” “toast” Furfurylthiol “roasted”

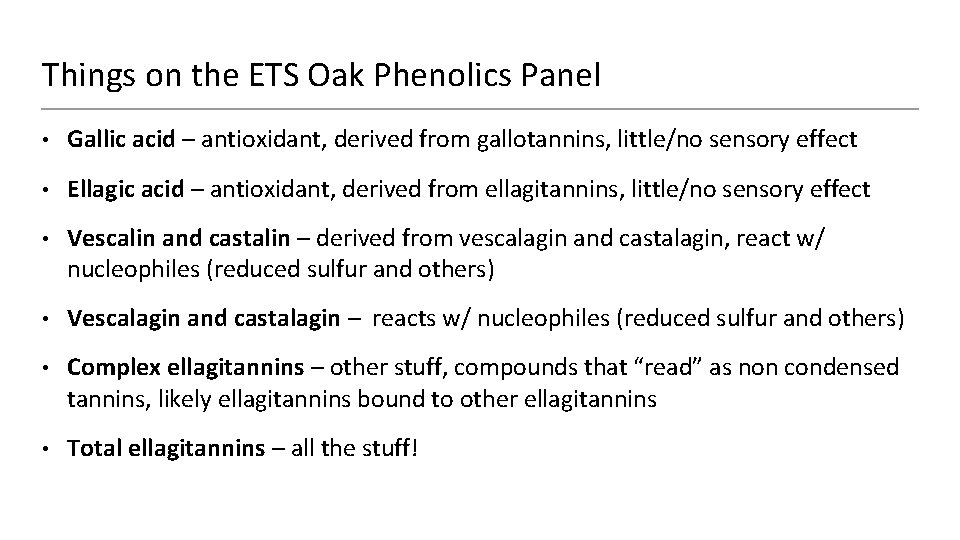
Things on the ETS Oak Phenolics Panel • Gallic acid – antioxidant, derived from gallotannins, little/no sensory effect • Ellagic acid – antioxidant, derived from ellagitannins, little/no sensory effect • Vescalin and castalin – derived from vescalagin and castalagin, react w/ nucleophiles (reduced sulfur and others) • Vescalagin and castalagin – reacts w/ nucleophiles (reduced sulfur and others) • Complex ellagitannins – other stuff, compounds that “read” as non condensed tannins, likely ellagitannins bound to other ellagitannins • Total ellagitannins – all the stuff!

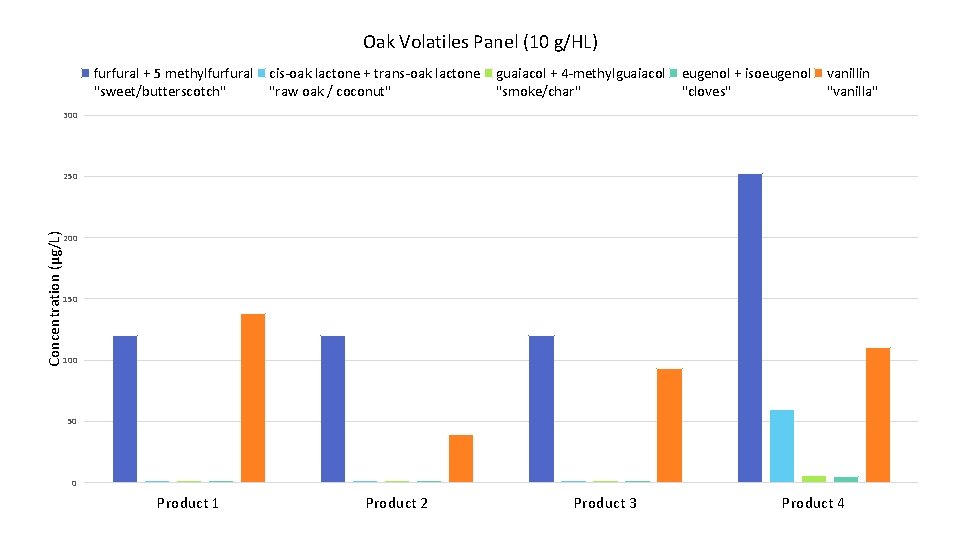
Things on the ETS Oak Aroma Panel • Furfural + 5 -methylfurfural – “butterscotch” “caramel” • cis-oak lactone + trans-oak lactone – “raw oak” “coconut” • Guaiacol + 4 -methylguaiacol – “charred” “smoky” “spicy” • Eugenol + Isoeugenol – “clove” • Vanillin – “vanilla”

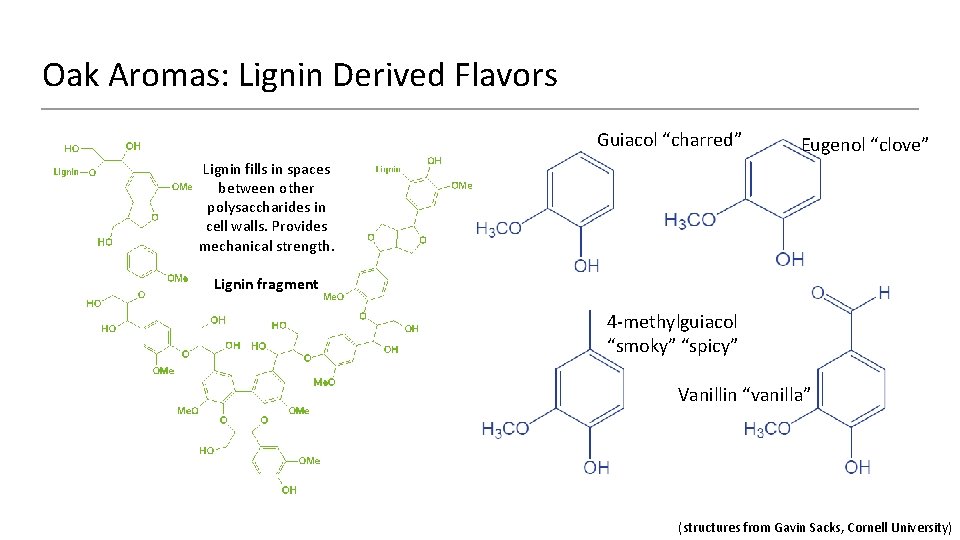
Oak Aromas: Lignin Derived Flavors Guiacol “charred” Eugenol “clove” Lignin fills in spaces between other polysaccharides in cell walls. Provides mechanical strength. Lignin fragment 4 -methylguiacol “smoky” “spicy” Vanillin “vanilla” (structures from Gavin Sacks, Cornell University)

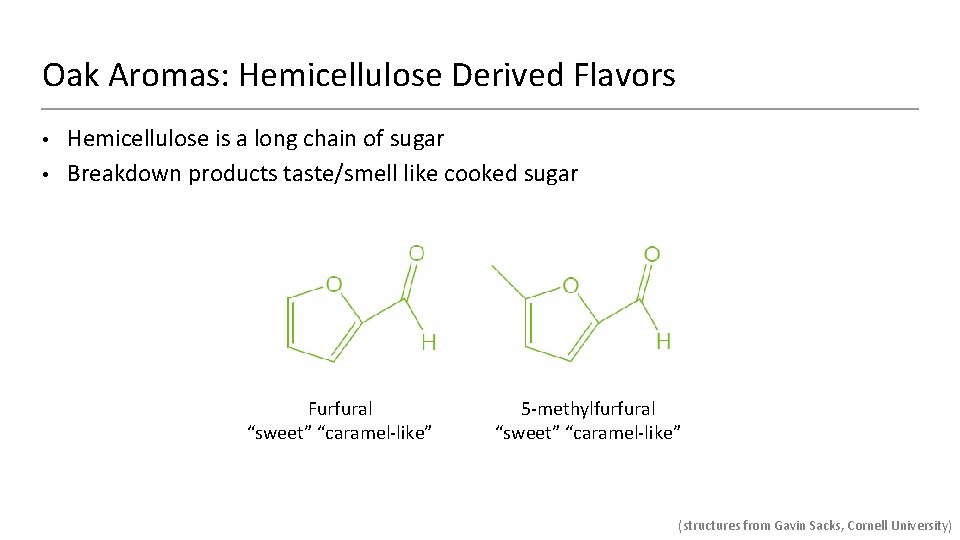
Oak Aromas: Hemicellulose Derived Flavors • • Hemicellulose is a long chain of sugar Breakdown products taste/smell like cooked sugar Furfural “sweet” “caramel-like” 5 -methylfurfural “sweet” “caramel-like” (structures from Gavin Sacks, Cornell University)

Let’s Look at Some Products Oak Phenolics and Oak Aroma Compounds in 4 Products

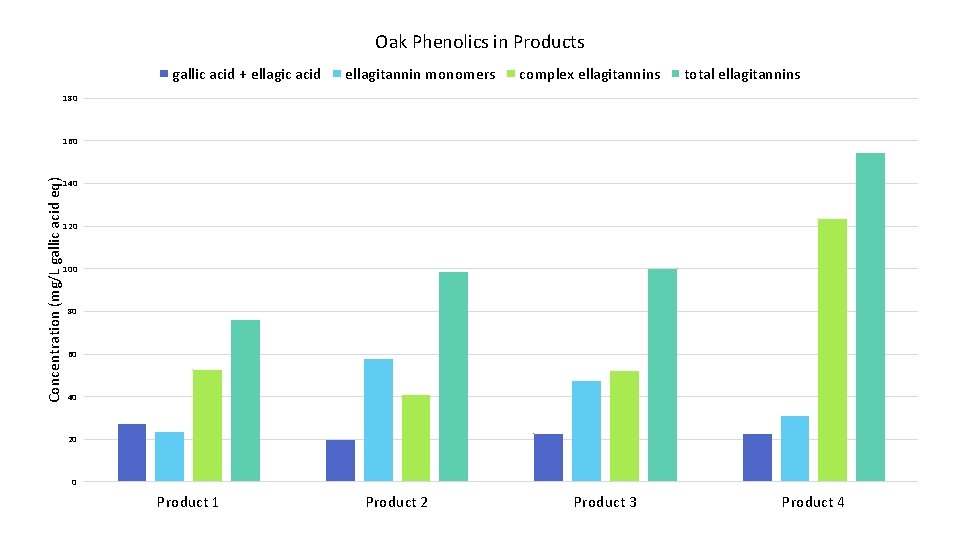
Oak Phenolics in Products gallic acid + ellagic acid ellagitannin monomers complex ellagitannins total ellagitannins 180 Concentration (mg/L gallic acid eq) 160 140 120 100 80 60 40 20 0 Product 1 Product 2 Product 3 Product 4

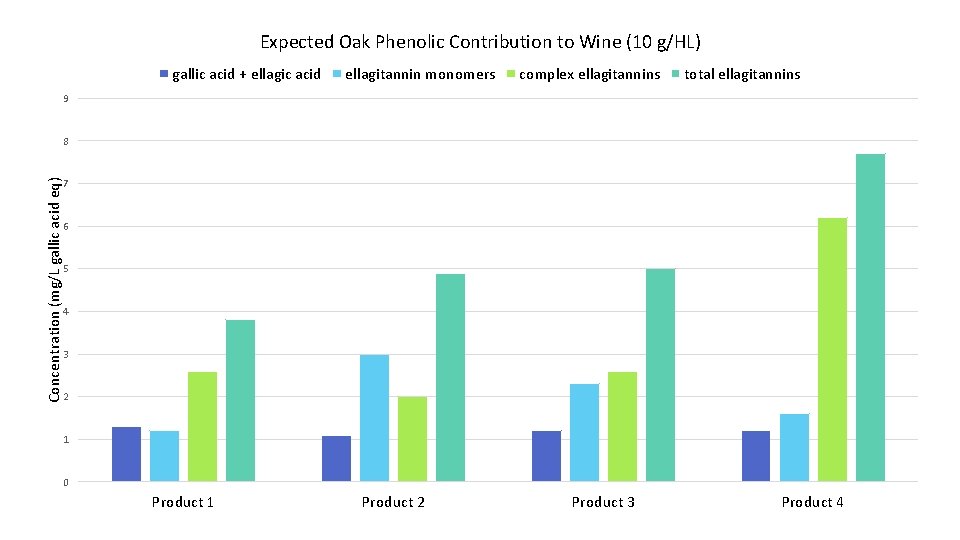
Expected Oak Phenolic Contribution to Wine (10 g/HL) gallic acid + ellagic acid ellagitannin monomers complex ellagitannins total ellagitannins 9 Concentration (mg/L gallic acid eq) 8 7 6 5 4 3 2 1 0 Product 1 Product 2 Product 3 Product 4

Oak Volatiles Panel (10 g/HL) furfural + 5 methylfurfural "sweet/butterscotch" cis-oak lactone + trans-oak lactone "raw oak / coconut" guaiacol + 4 -methylguaiacol "smoke/char" eugenol + isoeugenol "cloves" vanillin "vanilla" 300 Concentration (μg/L) 250 200 150 100 50 0 Product 1 Product 2 Product 3 Product 4

Odor Activity Values furfural + 5 methylfurfural "sweet/butterscotch" cis-oak lactone + trans-oak lactone "raw oak / coconut" guaiacol + 4 -methylguaiacol "smoke/char" eugenol + isoeugenol "cloves" vanillin "vanilla" 10 9 ← Value = 17 Importance of a compound to the odor of a sample OAV = concentration / detection threshold 8 Odor Activity Value 7 6 5 4 3 2 1 0 Product 1 Product 2 Product 3 Product 4 Detection thresholds from Chatonnet 1995

Thank You

References Chatonnet, P. 1995. Influence des proceeds de tonnellerie et des techniques d’élevage sur la composition et la qualité des vins élevés en fûts de chêne. Ph. D Thesis. Université de Bordeaux, FR. He, F. , Liang, N. , Mu, L. , Pan, Q. , Wang, J. Reeves, M. , and C. Duan. 2012. Anthocyanins and their variation in red wines I; monomeric anthocyanins and their color expression. Molecules. 17: 1571 -1601. He, F. , Liang, N. , Mu, L. , Pan, Q. , Wang, J. Reeves, M. , and C. Duan. 2012. Anthocyanins and their variation in red wines II; anthocyanin derived pigments and their color evolution. Molecules. 17: 1483 -1519. Kontoudakis, N. 2010. Grape phenolic maturity; Determination methods and consequences on wine phenolic composition. Thesis, Rovira i Virgili University, Tarragona. Loch, R. 2002. Micro-oxygenation: a large winery case study. In ASVO Proceedings of Seminar ‘Use of gases in winemaking’. Allen, M. (ed), pp. 45 -53. Australian Society for Viticulture and Oenology. Adelaide. Ribéreau-Gayon, P. , Glories, Y. , Maujean, A. , and D. Dubourdieu. 2006. Handbook of Enology Volume 2; The Chemistry of Wine Stabilization and Treatments. John Wiley & Sons Ltd, Chichester, United Kingdom. Waterhouse, A. 2002. Wine Phenolics. Ann. N. Y. Acad. Sci. 957: 21 -36.

Thank You to Our Session Sponsor:
 Phenolic molding compounds
Phenolic molding compounds Chemical germicides formulated for use on skin
Chemical germicides formulated for use on skin Tripod technology corporation
Tripod technology corporation Propagation
Propagation Tannins
Tannins Gelatin test for tannins
Gelatin test for tannins Tincture alkana test
Tincture alkana test Test for glycosides
Test for glycosides Example of tannins in pharmacognosy
Example of tannins in pharmacognosy Tannin wine
Tannin wine Growth development maturation
Growth development maturation Learning can be defined as
Learning can be defined as Hormonal stimulus
Hormonal stimulus Castles secrets mysteries and legends
Castles secrets mysteries and legends Gothic story and dark secrets
Gothic story and dark secrets Unit 4 development through the life stages
Unit 4 development through the life stages Is sunlight necessary for seed germination
Is sunlight necessary for seed germination What is the maturation theory
What is the maturation theory Affinity maturation somatic hypermutation
Affinity maturation somatic hypermutation Gessel theory
Gessel theory Examples of internal validity
Examples of internal validity