Phase III SINDAS Interim Analysis of Firstline EGFR


- Slides: 10

Phase III SINDAS: Interim Analysis of First-line EGFR TKI With vs Without SBRT in Patients With EGFR-Mutated Oligometastatic NSCLC CCO Independent Conference Coverage* Highlights of the 2020 ASCO Virtual Scientific Meeting, May 29 -31, 2020 *CCO is an independent medical education company that provides state-of-the-art medical information to healthcare professionals through conference coverage and other educational programs. This activity is provided by Clinical Care Options, LLC Supported by educational grants from Astra. Zeneca; Daiichi Sankyo, Inc. ; Ipsen Pharma; Jazz Pharmaceuticals, Inc. ; and Merck Sharp & Dohme Corp.

About These Slides § Please feel free to use, update, and share some or all of these slides in your noncommercial presentations to colleagues or patients § When using our slides, please retain the source attribution: Slide credit: clinicaloptions. com § These slides may not be published, posted online, or used in commercial presentations without permission. Please contact permissions@clinicaloptions. com for details

SINDAS Interim Analysis: Background § Oligometastatic NSCLC generally defined as disease comprising a primary tumor and limited number of synchronous or metachronous metastases (eg, 1 -5)[1, 2] § Patients with oligometastatic NSCLC may benefit from ablation of metastases via local consolidative therapy with surgery ± radiation[3] ‒ In a randomized phase II study, local consolidative therapy + So. C maintenance treatment, including observation, significantly prolonged PFS vs maintenance alone in patients with NSCLC and 1 -3 oligometastases after first-line systemic therapy ‒ Median PFS: 11. 9 vs 3. 9 mos (HR: 0. 35; P =. 005) § Current interim analysis compared efficacy, safety of first-line EGFR TKI with vs without SBRT in Chinese patients with EGFR-mutated oligometastatic NSCLC enrolled on phase III SINDAS trial[4] 1. Richard. Lung Cancer (Auckl). 2016; 7: 129. 2. Schanne. Cancer Treat Rev. 2019; 80: 101892. 3. Gomez. Lancet Oncol. 2016; 17: 1672. 4. Wang. ASCO 2020. Abstr 9508. Slide credit: clinicaloptions. com

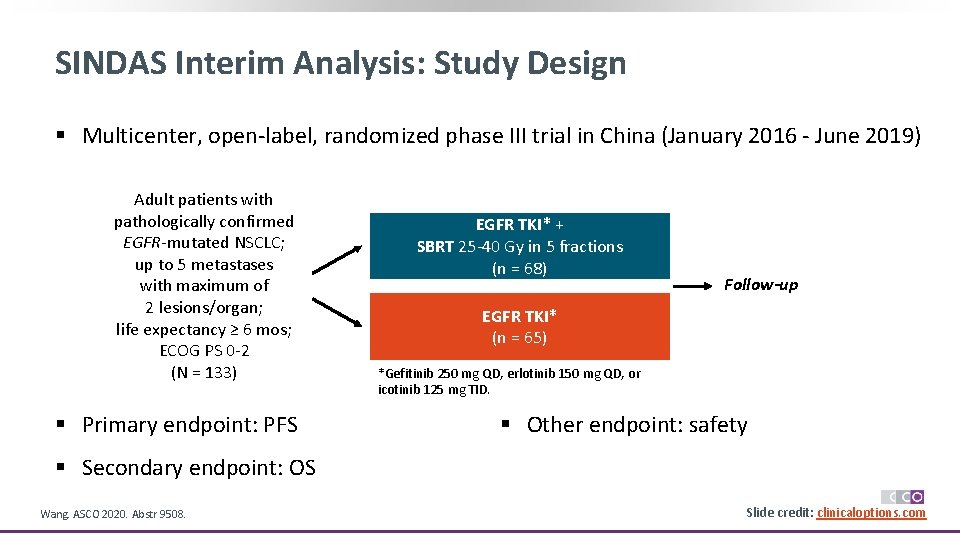
SINDAS Interim Analysis: Study Design § Multicenter, open-label, randomized phase III trial in China (January 2016 - June 2019) Adult patients with pathologically confirmed EGFR-mutated NSCLC; up to 5 metastases with maximum of 2 lesions/organ; life expectancy ≥ 6 mos; ECOG PS 0 -2 (N = 133) § Primary endpoint: PFS EGFR TKI* + SBRT 25 -40 Gy in 5 fractions (n = 68) Follow-up EGFR TKI* (n = 65) *Gefitinib 250 mg QD, erlotinib 150 mg QD, or icotinib 125 mg TID. § Other endpoint: safety § Secondary endpoint: OS Wang. ASCO 2020. Abstr 9508. Slide credit: clinicaloptions. com

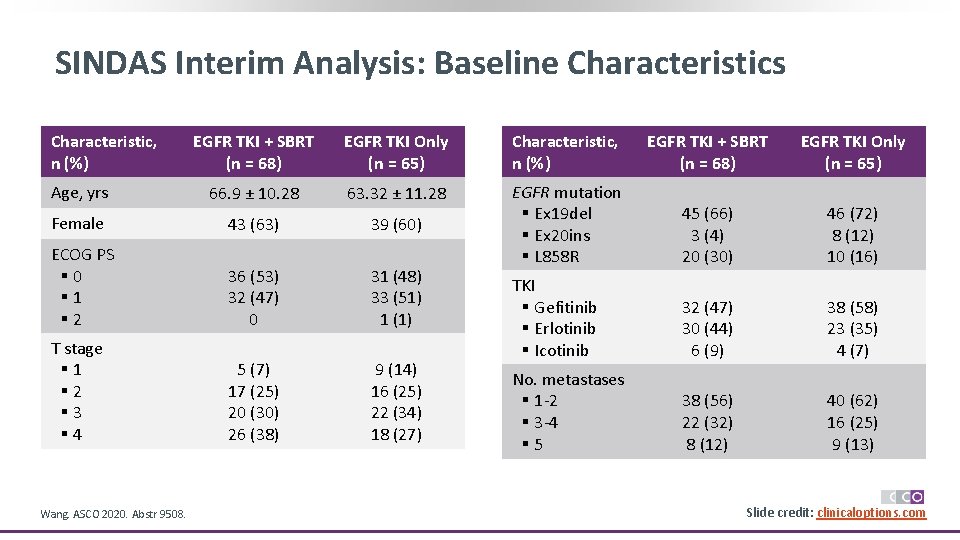
SINDAS Interim Analysis: Baseline Characteristics Characteristic, n (%) EGFR TKI + SBRT (n = 68) EGFR TKI Only (n = 65) Age, yrs 66. 9 ± 10. 28 63. 32 ± 11. 28 Female 43 (63) 39 (60) ECOG PS § 0 § 1 § 2 36 (53) 32 (47) 0 31 (48) 33 (51) 1 (1) EGFR mutation § Ex 19 del § Ex 20 ins § L 858 R 45 (66) 3 (4) 20 (30) 46 (72) 8 (12) 10 (16) T stage § 1 § 2 § 3 § 4 5 (7) 17 (25) 20 (30) 26 (38) 9 (14) 16 (25) 22 (34) 18 (27) TKI § Gefitinib § Erlotinib § Icotinib 32 (47) 30 (44) 6 (9) 38 (58) 23 (35) 4 (7) No. metastases § 1 -2 § 3 -4 § 5 38 (56) 22 (32) 8 (12) 40 (62) 16 (25) 9 (13) Wang. ASCO 2020. Abstr 9508. Slide credit: clinicaloptions. com

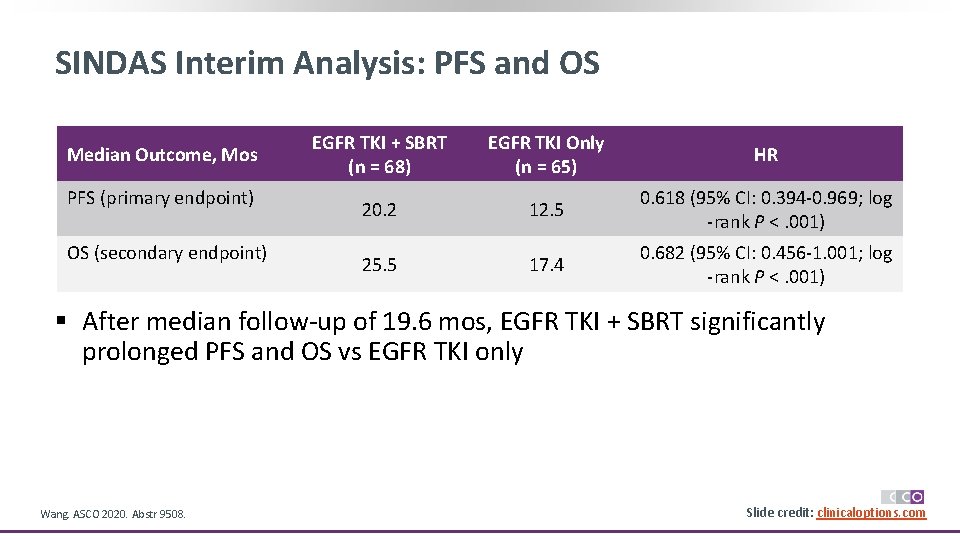
SINDAS Interim Analysis: PFS and OS Median Outcome, Mos PFS (primary endpoint) OS (secondary endpoint) EGFR TKI + SBRT (n = 68) EGFR TKI Only (n = 65) HR 20. 2 12. 5 0. 618 (95% CI: 0. 394 -0. 969; log -rank P <. 001) 25. 5 17. 4 0. 682 (95% CI: 0. 456 -1. 001; log -rank P <. 001) § After median follow-up of 19. 6 mos, EGFR TKI + SBRT significantly prolonged PFS and OS vs EGFR TKI only Wang. ASCO 2020. Abstr 9508. Slide credit: clinicaloptions. com

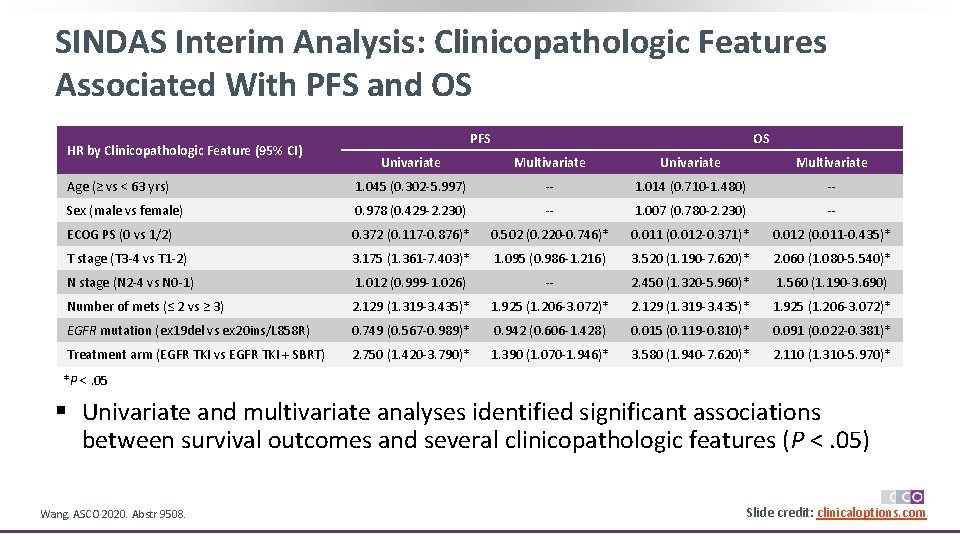
SINDAS Interim Analysis: Clinicopathologic Features Associated With PFS and OS HR by Clinicopathologic Feature (95% CI) PFS OS Univariate Multivariate Age (≥ vs < 63 yrs) 1. 045 (0. 302 -5. 997) -- 1. 014 (0. 710 -1. 480) -- Sex (male vs female) 0. 978 (0. 429 -2. 230) -- 1. 007 (0. 780 -2. 230) -- ECOG PS (0 vs 1/2) 0. 372 (0. 117 -0. 876)* 0. 502 (0. 220 -0. 746)* 0. 011 (0. 012 -0. 371)* 0. 012 (0. 011 -0. 435)* T stage (T 3 -4 vs T 1 -2) 3. 175 (1. 361 -7. 403)* 1. 095 (0. 986 -1. 216) 3. 520 (1. 190 -7. 620)* 2. 060 (1. 080 -5. 540)* N stage (N 2 -4 vs N 0 -1) 1. 012 (0. 999 -1. 026) -- 2. 450 (1. 320 -5. 960)* 1. 560 (1. 190 -3. 690) Number of mets (≤ 2 vs ≥ 3) 2. 129 (1. 319 -3. 435)* 1. 925 (1. 206 -3. 072)* EGFR mutation (ex 19 del vs ex 20 ins/L 858 R) 0. 749 (0. 567 -0. 989)* 0. 942 (0. 606 -1. 428) 0. 015 (0. 119 -0. 810)* 0. 091 (0. 022 -0. 381)* Treatment arm (EGFR TKI vs EGFR TKI + SBRT) 2. 750 (1. 420 -3. 790)* 1. 390 (1. 070 -1. 946)* 3. 580 (1. 940 -7. 620)* 2. 110 (1. 310 -5. 970)* *P <. 05 § Univariate and multivariate analyses identified significant associations between survival outcomes and several clinicopathologic features (P <. 05) Wang. ASCO 2020. Abstr 9508. Slide credit: clinicaloptions. com

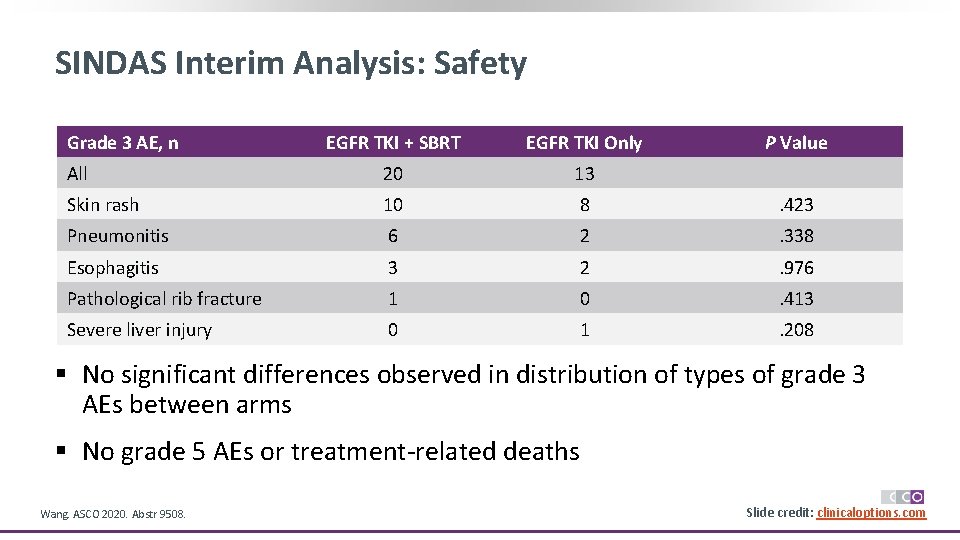
SINDAS Interim Analysis: Safety Grade 3 AE, n EGFR TKI + SBRT EGFR TKI Only P Value All 20 13 Skin rash 10 8 . 423 Pneumonitis 6 2 . 338 Esophagitis 3 2 . 976 Pathological rib fracture 1 0 . 413 Severe liver injury 0 1 . 208 § No significant differences observed in distribution of types of grade 3 AEs between arms § No grade 5 AEs or treatment-related deaths Wang. ASCO 2020. Abstr 9508. Slide credit: clinicaloptions. com

SINDAS Interim Analysis: Conclusions § In this interim analysis of the phase III SINDAS trial, the addition of upfront SBRT to first-line EGFR TKI significantly prolonged PFS and OS vs EGFR TKI alone in Chinese patients with EGFR-mutated oligometastatic NSCLC ‒ Median PFS (primary endpoint): 20. 2 vs 12. 5 mos (HR: 0. 618; P <. 001) ‒ Median OS (secondary endpoint): 25. 5 vs 17. 4 mos (HR: 0. 682; P <. 001) § Safety profiles generally comparable between treatment arms § Investigators concluded that findings support beneficial use of consolidative SBRT for oligometastatic NSCLC ‒ Suggest further evaluation of upfront aggressive local treatment of metastases at diagnosis in large phase III trials Wang. ASCO 2020. Abstr 9508. Slide credit: clinicaloptions. com

Go Online for More CCO Coverage of ASCO 2020! Short slideset summaries and additional CME-certified analyses with expert faculty commentary on key studies in: § Breast cancer § Gynecologic cancers § Gastrointestinal cancers § Hematologic malignancies § Genitourinary cancers § Lung cancer clinicaloptions. com/oncology