p H scale Logarithmic scale expressing H1 concentration


![p. H scale • Logarithmic scale –expressing H+1 concentration, [H+1] • If p. H p. H scale • Logarithmic scale –expressing H+1 concentration, [H+1] • If p. H](https://slidetodoc.com/presentation_image_h2/63e76686113cd6eabda007190285e9a2/image-3.jpg)

![Molarity to p. H To determine p. H: • Express [H+1] in scientific notation Molarity to p. H To determine p. H: • Express [H+1] in scientific notation](https://slidetodoc.com/presentation_image_h2/63e76686113cd6eabda007190285e9a2/image-5.jpg)
![p. H [H+1] p. H 1 M or 1 X 100 M 0 0. p. H [H+1] p. H 1 M or 1 X 100 M 0 0.](https://slidetodoc.com/presentation_image_h2/63e76686113cd6eabda007190285e9a2/image-7.jpg)

![p. H to Molarity p. H = -log[H+1], solve for [H+1] • -p. H p. H to Molarity p. H = -log[H+1], solve for [H+1] • -p. H](https://slidetodoc.com/presentation_image_h2/63e76686113cd6eabda007190285e9a2/image-9.jpg)
![p. OH • • By analogy: p. OH is defined as –log[OH-1] Express [OH-1] p. OH • • By analogy: p. OH is defined as –log[OH-1] Express [OH-1]](https://slidetodoc.com/presentation_image_h2/63e76686113cd6eabda007190285e9a2/image-10.jpg)
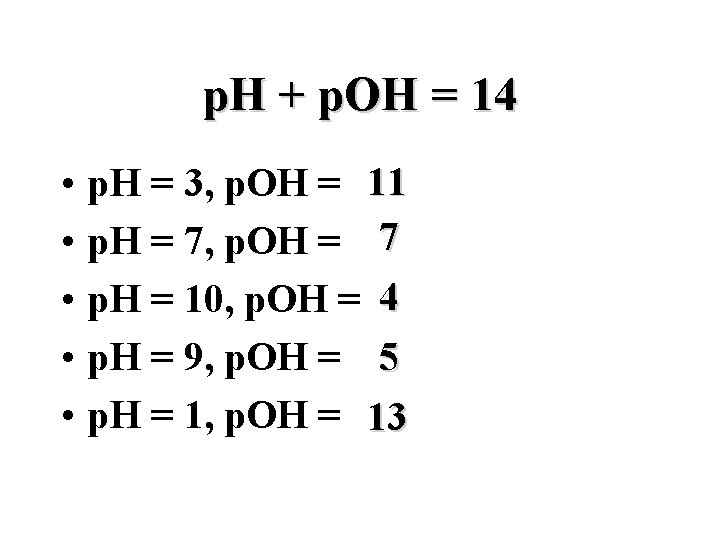

![ACID or BASE? • Acids: [H+1] [OH-1] • Bases: [OH-1] [H+1] ACID or BASE? • Acids: [H+1] [OH-1] • Bases: [OH-1] [H+1]](https://slidetodoc.com/presentation_image_h2/63e76686113cd6eabda007190285e9a2/image-13.jpg)
![• If p. H = 4 • [H+1] = ? Antilog(-p. H) = • If p. H = 4 • [H+1] = ? Antilog(-p. H) =](https://slidetodoc.com/presentation_image_h2/63e76686113cd6eabda007190285e9a2/image-16.jpg)
![• If the [OH-1] = 1 X 10 -3 • p. OH = • If the [OH-1] = 1 X 10 -3 • p. OH =](https://slidetodoc.com/presentation_image_h2/63e76686113cd6eabda007190285e9a2/image-17.jpg)
![• If the +1 [H ] =1 x -5 10 M • The • If the +1 [H ] =1 x -5 10 M • The](https://slidetodoc.com/presentation_image_h2/63e76686113cd6eabda007190285e9a2/image-18.jpg)
![[H+] (M) p. H [OH-] (M) 1 x 10 -3 3 1 x 10 [H+] (M) p. H [OH-] (M) 1 x 10 -3 3 1 x 10](https://slidetodoc.com/presentation_image_h2/63e76686113cd6eabda007190285e9a2/image-19.jpg)





- Slides: 24


![p H scale Logarithmic scale expressing H1 concentration H1 If p H p. H scale • Logarithmic scale –expressing H+1 concentration, [H+1] • If p. H](https://slidetodoc.com/presentation_image_h2/63e76686113cd6eabda007190285e9a2/image-3.jpg)
p. H scale • Logarithmic scale –expressing H+1 concentration, [H+1] • If p. H changes by factor of 1, [H+1] changes by factor of 10 • p. H = -log[H+1]

![Molarity to p H To determine p H Express H1 in scientific notation Molarity to p. H To determine p. H: • Express [H+1] in scientific notation](https://slidetodoc.com/presentation_image_h2/63e76686113cd6eabda007190285e9a2/image-5.jpg)
Molarity to p. H To determine p. H: • Express [H+1] in scientific notation • Remember, [ ] means concentration of whatever is inside brackets • The log is the power of 10

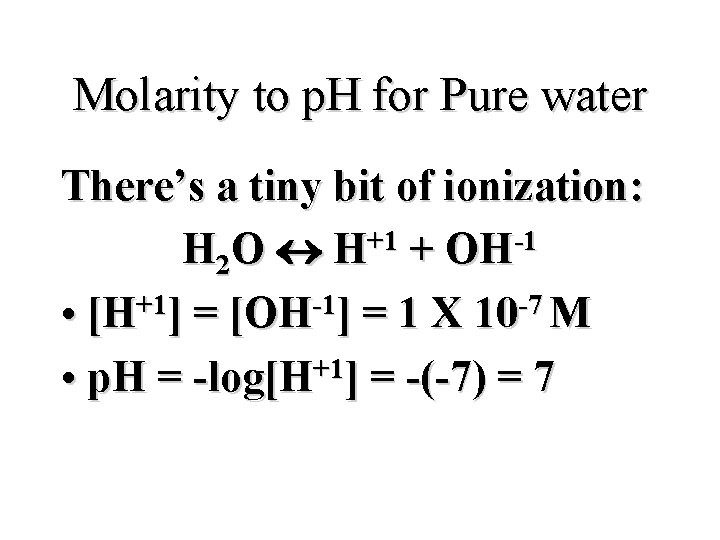
Molarity to p. H for Pure water There’s a tiny bit of ionization: H 2 O H+1 + OH-1 • [H+1] = [OH-1] = 1 X 10 -7 M • p. H = -log[H+1] = -(-7) = 7
![p H H1 p H 1 M or 1 X 100 M 0 0 p. H [H+1] p. H 1 M or 1 X 100 M 0 0.](https://slidetodoc.com/presentation_image_h2/63e76686113cd6eabda007190285e9a2/image-7.jpg)
p. H [H+1] p. H 1 M or 1 X 100 M 0 0. 1 M 0. 01 M. 001 M or or Or 1 X 10 -1 1 X 10 -2 1 X 10 -3 M M M 1 2 3

![p H to Molarity p H logH1 solve for H1 p H p. H to Molarity p. H = -log[H+1], solve for [H+1] • -p. H](https://slidetodoc.com/presentation_image_h2/63e76686113cd6eabda007190285e9a2/image-9.jpg)
p. H to Molarity p. H = -log[H+1], solve for [H+1] • -p. H = log[H+1] • Antilog(-p. H) = [H+1] • Say p. H = 5, then –p. H = -5 • Antilog(-5) = 10 -5 • The –p. H becomes the power of 10!
![p OH By analogy p OH is defined as logOH1 Express OH1 p. OH • • By analogy: p. OH is defined as –log[OH-1] Express [OH-1]](https://slidetodoc.com/presentation_image_h2/63e76686113cd6eabda007190285e9a2/image-10.jpg)
p. OH • • By analogy: p. OH is defined as –log[OH-1] Express [OH-1] in scientific notation If [OH-1] = 1. 0 X 10 -3 M Then p. OH = -log(10 -3) = -(-3) = 3
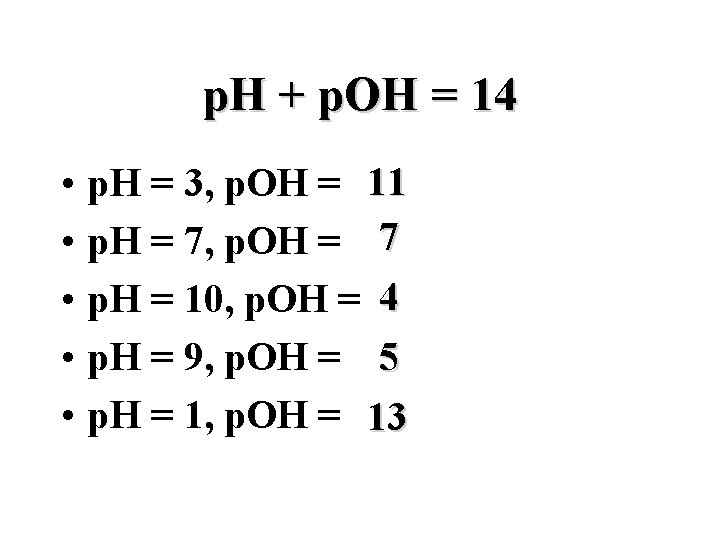
p. H + p. OH = 14 • • • p. H = 3, p. OH = 11 p. H = 7, p. OH = 7 p. H = 10, p. OH = 4 p. H = 9, p. OH = 5 p. H = 1, p. OH = 13

![ACID or BASE Acids H1 OH1 Bases OH1 H1 ACID or BASE? • Acids: [H+1] [OH-1] • Bases: [OH-1] [H+1]](https://slidetodoc.com/presentation_image_h2/63e76686113cd6eabda007190285e9a2/image-13.jpg)
ACID or BASE? • Acids: [H+1] [OH-1] • Bases: [OH-1] [H+1]


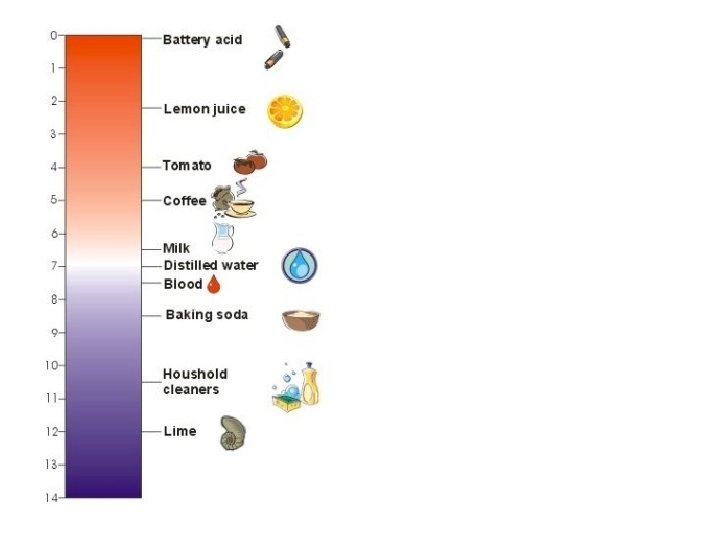
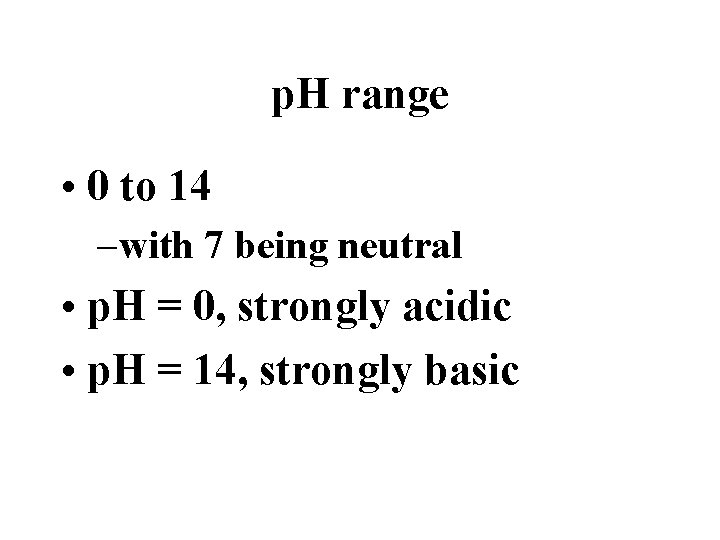
p. H range • 0 to 14 – with 7 being neutral • p. H = 0, strongly acidic • p. H = 14, strongly basic
![If p H 4 H1 Antilogp H • If p. H = 4 • [H+1] = ? Antilog(-p. H) =](https://slidetodoc.com/presentation_image_h2/63e76686113cd6eabda007190285e9a2/image-16.jpg)
• If p. H = 4 • [H+1] = ? Antilog(-p. H) = [H+1] Antilog(-4) = 1 x 10 -4 M • p. OH = ? 10 • [OH-1] Antilog(-10) = [OH-1] 1 x 10 -10 M = [OH-1] =?
![If the OH1 1 X 10 3 p OH • If the [OH-1] = 1 X 10 -3 • p. OH =](https://slidetodoc.com/presentation_image_h2/63e76686113cd6eabda007190285e9a2/image-17.jpg)
• If the [OH-1] = 1 X 10 -3 • p. OH = ? • p. H = ? • +1 [H ] p. OH = -log[OH-1] = -log(10 -3) = -(-3) = 3 14 – 3 = 11 =? Antilog(-11) = 1 x 10 -11 M
![If the 1 H 1 x 5 10 M The • If the +1 [H ] =1 x -5 10 M • The](https://slidetodoc.com/presentation_image_h2/63e76686113cd6eabda007190285e9a2/image-18.jpg)
• If the +1 [H ] =1 x -5 10 M • The p. H = ? 5 • The p. OH = ? 14 – 5 = 9 • The [OH-] = ? Antilog(-9) = 1 x 10 -9 M
![H M p H OH M 1 x 10 3 3 1 x 10 [H+] (M) p. H [OH-] (M) 1 x 10 -3 3 1 x 10](https://slidetodoc.com/presentation_image_h2/63e76686113cd6eabda007190285e9a2/image-19.jpg)
[H+] (M) p. H [OH-] (M) 1 x 10 -3 3 1 x 10 -11 p. OH Acidic or Basic 11 A 9 1 x 10 -5 5 B 1 x 10 -2 2 1 x 10 -12 12 A 1 x 10 -8 8 1 x 10 -6 6 B 1 x 10 -9

How to safely test p. H • Instruments – use a p. H meter • Indicators – use a series of indicators • See if the substance reacts with a metal other than Cu, Ag, or Au • NEVER “taste”


Indicator • substance that changes color over narrow p. H range • Use several indicators to narrow down p. H range of substance

