Other units of concentration Dilution Equation MV MV







- Slides: 15

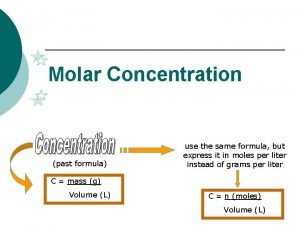
Other units of concentration

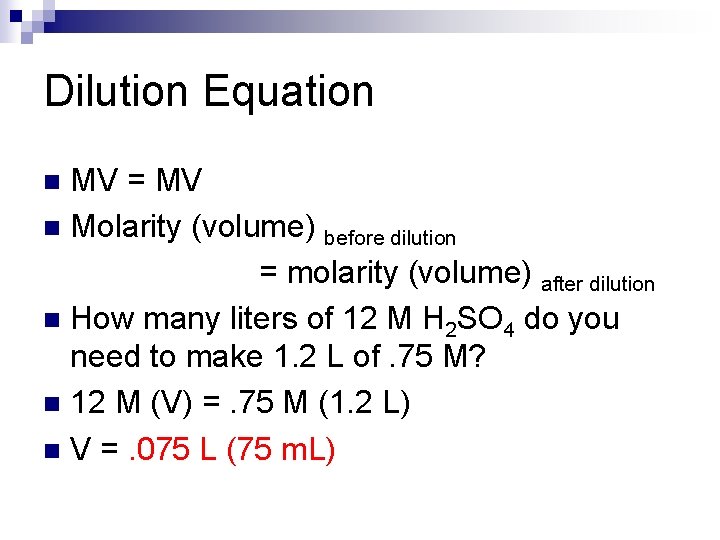
Dilution Equation MV = MV n Molarity (volume) before dilution = molarity (volume) after dilution n How many liters of 12 M H 2 SO 4 do you need to make 1. 2 L of. 75 M? n 12 M (V) =. 75 M (1. 2 L) n V =. 075 L (75 m. L) n

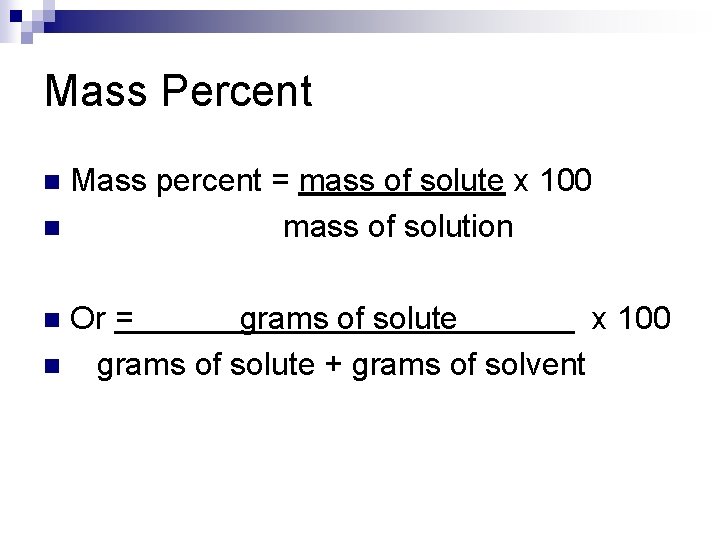
Mass Percent Mass percent = mass of solute x 100 n mass of solution n Or = grams of solute x 100 n grams of solute + grams of solvent n

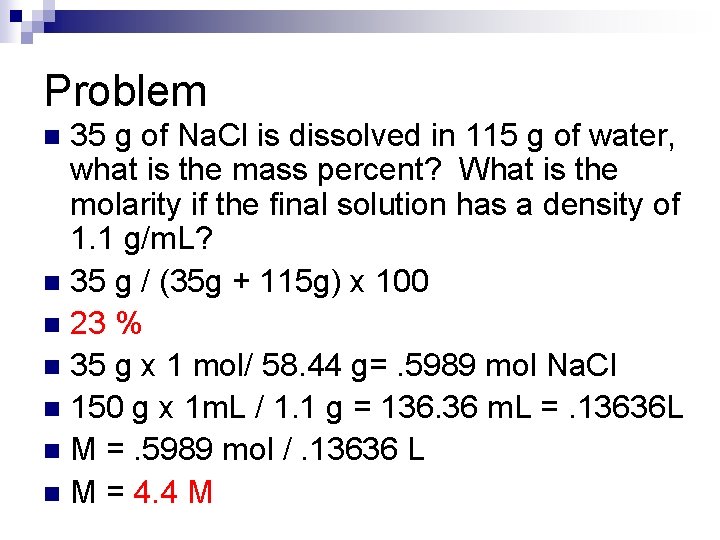
Problem 35 g of Na. Cl is dissolved in 115 g of water, what is the mass percent? What is the molarity if the final solution has a density of 1. 1 g/m. L? n 35 g / (35 g + 115 g) x 100 n 23 % n 35 g x 1 mol/ 58. 44 g=. 5989 mol Na. Cl n 150 g x 1 m. L / 1. 1 g = 136. 36 m. L =. 13636 L n M =. 5989 mol /. 13636 L n M = 4. 4 M n

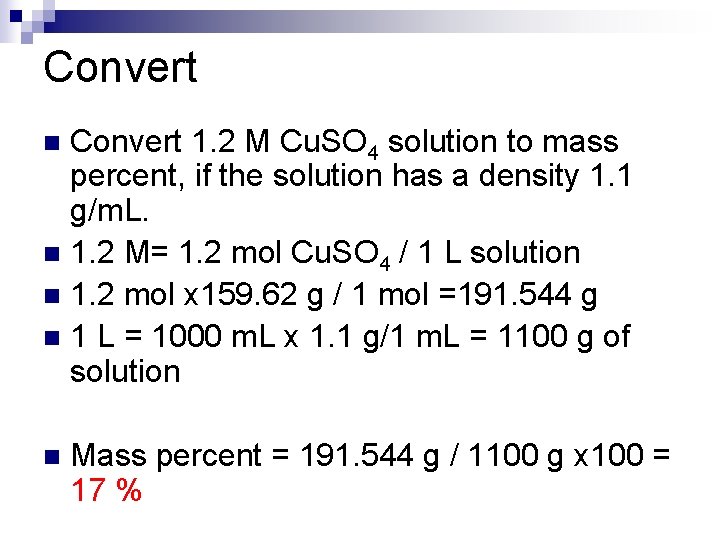
Convert 1. 2 M Cu. SO 4 solution to mass percent, if the solution has a density 1. 1 g/m. L. n 1. 2 M= 1. 2 mol Cu. SO 4 / 1 L solution n 1. 2 mol x 159. 62 g / 1 mol =191. 544 g n 1 L = 1000 m. L x 1. 1 g/1 m. L = 1100 g of solution n n Mass percent = 191. 544 g / 1100 g x 100 = 17 %

More practice n 55 g of Ca. Cl 2 is dissolved in 115 g of water, what is the mass percent? What is the molarity if the final solution has a density of 1. 1 g/m. L?


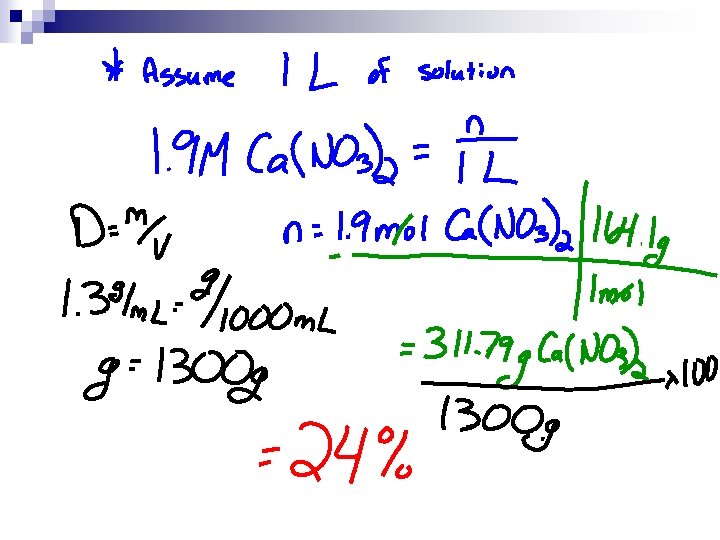
More n Convert 1. 9 M Ca(NO 3)2 solution to mass percent, if the solution has a density 1. 3 g/m. L.


Parts per thousand/million etc. Mass percent can also be called parts per hundred (although it never is) n Parts per thousand is the same as mass percent except instead of multiplying by a 100 you multiply by 1000. n Parts per million is multiplied by 1, 000 n Pollen counts are normally reported in this n

Mole Fraction

Mole Fraction or molar fraction is represented by the Greek letter chi, n = mole solute/mole solution n

Convert a 2. 3 M of water solution of aluminum nitrate Al(NO 3)3 to mole fraction of aluminum nitrate if the density of the solution is 1. 14 g/m. L. n 2. 3 M = 2. 3 mol Al(NO 3)3 /1 L solution n 2. 3 mol x 213. 01 g/ 1 mol = 489. 923 g Al(NO 3)3 n 1 L = 1000 m. L x 1. 14 g/1 m. L = 1140 g solution n 1140 g - 489. 923 g = 650. 077 g water n 650. 077 g x 1 mol /18. 016 g = 36. 0833 mol n = 2. 3 mol/ (2. 3+36. 0833) mol =. 060 n

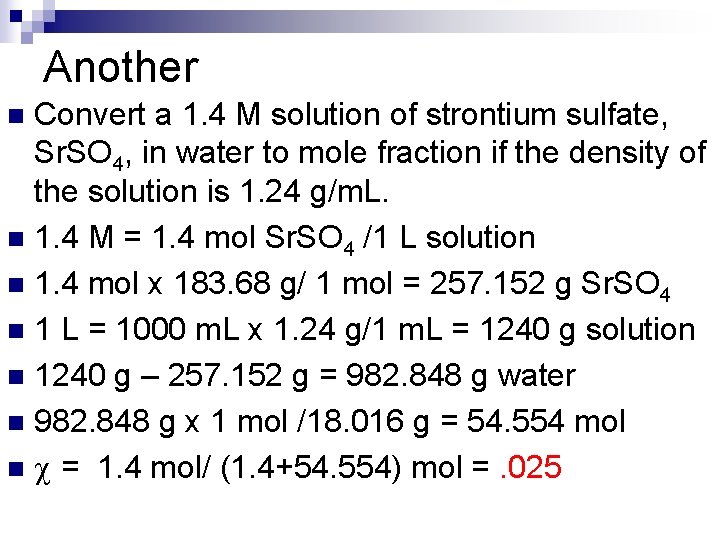
Another Convert a 1. 4 M solution of strontium sulfate, Sr. SO 4, in water to mole fraction if the density of the solution is 1. 24 g/m. L. n 1. 4 M = 1. 4 mol Sr. SO 4 /1 L solution n 1. 4 mol x 183. 68 g/ 1 mol = 257. 152 g Sr. SO 4 n 1 L = 1000 m. L x 1. 24 g/1 m. L = 1240 g solution n 1240 g – 257. 152 g = 982. 848 g water n 982. 848 g x 1 mol /18. 016 g = 54. 554 mol n = 1. 4 mol/ (1. 4+54. 554) mol =. 025 n

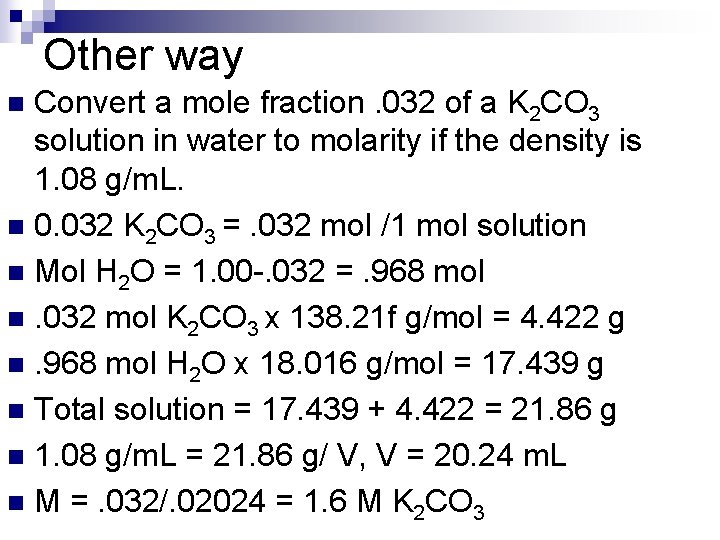
Other way Convert a mole fraction. 032 of a K 2 CO 3 solution in water to molarity if the density is 1. 08 g/m. L. n 0. 032 K 2 CO 3 =. 032 mol /1 mol solution n Mol H 2 O = 1. 00 -. 032 =. 968 mol n. 032 mol K 2 CO 3 x 138. 21 f g/mol = 4. 422 g n. 968 mol H 2 O x 18. 016 g/mol = 17. 439 g n Total solution = 17. 439 + 4. 422 = 21. 86 g n 1. 08 g/m. L = 21. 86 g/ V, V = 20. 24 m. L n M =. 032/. 02024 = 1. 6 M K 2 CO 3 n
 Dilution formula
Dilution formula Doubling dilution
Doubling dilution Serial dilution
Serial dilution Alligation pharmacy
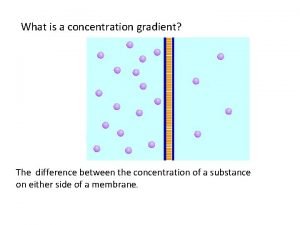
Alligation pharmacy Concentration gradient vs concentration difference
Concentration gradient vs concentration difference Movement of high concentration to low concentration
Movement of high concentration to low concentration Unit of concentration in chemistry
Unit of concentration in chemistry Units of concentration
Units of concentration Molarity units
Molarity units Dilution equation
Dilution equation Dilution ventilation formula
Dilution ventilation formula Dilution equation
Dilution equation K
K When units manufactured exceed units sold:
When units manufactured exceed units sold: How to find grams to moles
How to find grams to moles L
L