Osmosis and Diffusion CELL TRANSPORT Diffusion The net





- Slides: 10

Osmosis and Diffusion CELL TRANSPORT

Diffusion � The net movement of particles from an area of high concentration to an are of low concentration

Cells � Video �http: //www. youtube. com/watch? v=Ow 0 j. H 2 E g 8 v 4&feature=related&safety_mode=true&p ersist_safety_mode=1&safe=active � Dynamic equilibrium � Continuous movement without an overall change � Homeostasis �Regulation of an internal environment to maintain life

Cell membrane � selectively permeable � some materials can easily pass through and others cannot � Videos �http: //www. youtube. com/watch? v=Rl 5 Em. UQ dku. I&feature=related&safety_mode=true&pe rsist_safety_mode=1&safe=active �http: //www. youtube. com/watch? v=j 5 Qway 4 L Akk&feature=related&safety_mode=true&pe rsist_safety_mode=1&safe=active

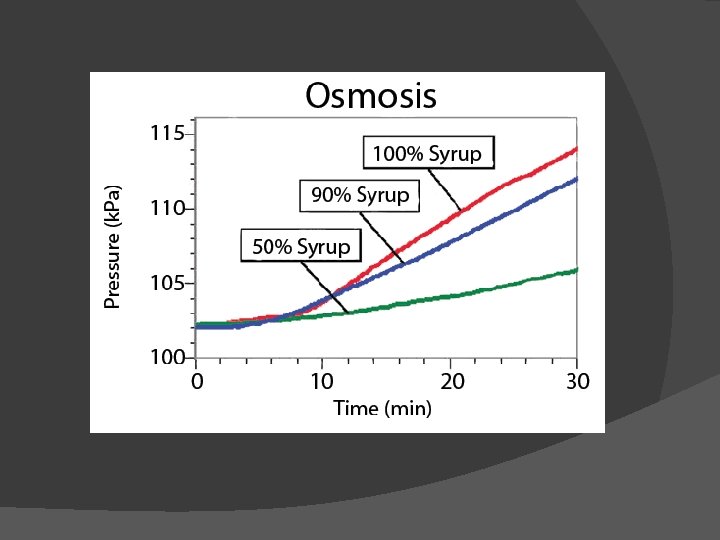
Osmosis � Diffusion of water across a selectively permeable membrane

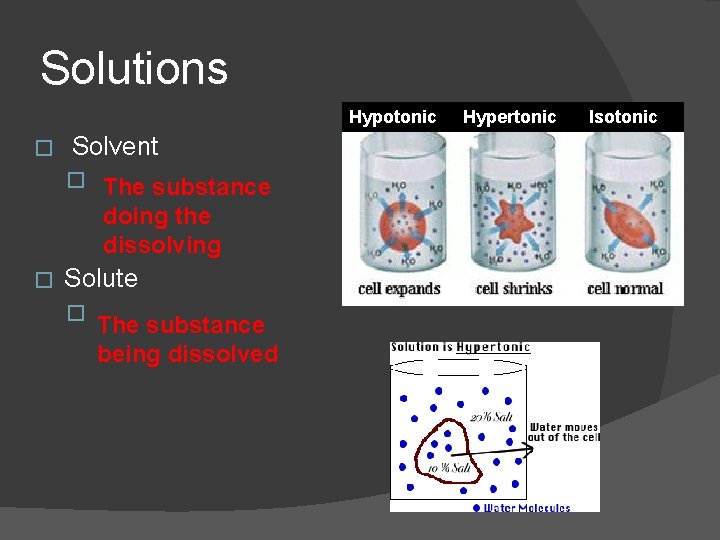
Solutions Hypotonic � Solvent � The substance doing the dissolving � Solute � The substance being dissolved Hypertonic Isotonic

Solutions � Isotonic � Water molecules move into and out of the cell at the same rate, and cells retain normal shape. Solution outside the cell has same solute concentration as inside the cell. � Hypertonic Water leaves a cell by osmosis causing the cell to shrink. Solution outside of cell has higher solute concentration than inside. � Hypotonic � Water enters cell by osmosis, causing it to swell. Solution outside of cell has lower solute concentration than the inside.


Osmosis and Potato Lab � Describe potato in water � Describe potato in salt water � Describe in terms of osmosis � Hypertonic, hypotonic, isotonic, semipermeable membrane, etc.

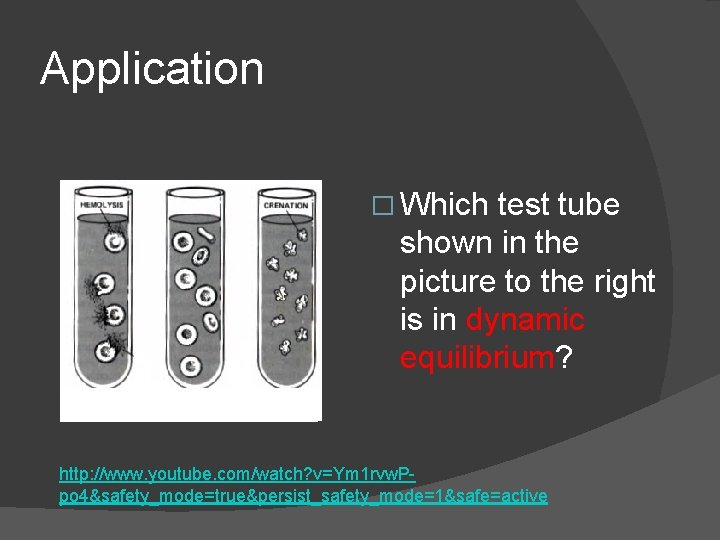
Application � Which test tube shown in the picture to the right is in dynamic equilibrium? http: //www. youtube. com/watch? v=Ym 1 rvw. Ppo 4&safety_mode=true&persist_safety_mode=1&safe=active