National Cheng Kung University Department of Resource Engineering




- Slides: 12

National Cheng Kung University Department of Resource Engineering Controlling the Morphology and Crystallite Size of Cu(In 0. 7 Ga 0. 3)Se 2 Nano-crystals Synthesized using a Heating-Up Method Student : Wei-Hsiang Hsu Advisor: Hsing-I Hsiang November 2012

Outline • • Introduction ★ The properties of CIGS ★ Objective Experimental Results and discussion Conclusion

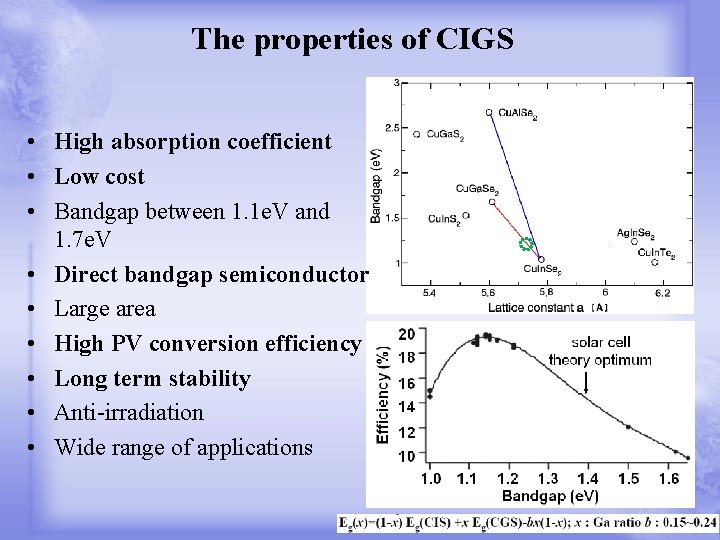
The properties of CIGS • High absorption coefficient • Low cost • Bandgap between 1. 1 e. V and 1. 7 e. V • Direct bandgap semiconductor • Large area • High PV conversion efficiency • Long term stability • Anti-irradiation • Wide range of applications

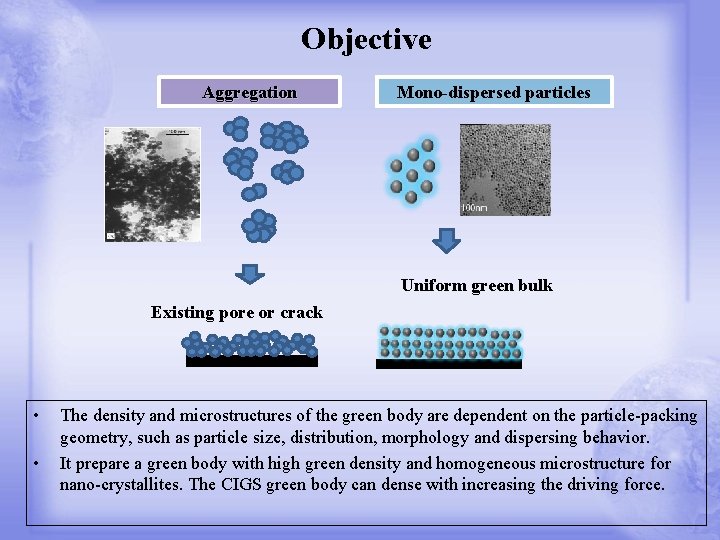
Objective Aggregation Mono-dispersed particles Uniform green bulk Existing pore or crack • • The density and microstructures of the green body are dependent on the particle-packing geometry, such as particle size, distribution, morphology and dispersing behavior. It prepare a green body with high green density and homogeneous microstructure for nano-crystallites. The CIGS green body can dense with increasing the driving force.

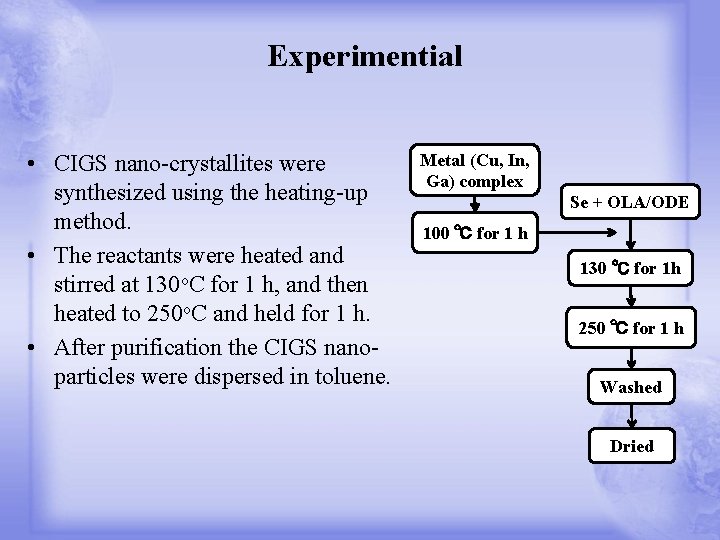
Experimential • CIGS nano-crystallites were synthesized using the heating-up method. • The reactants were heated and stirred at 130 o. C for 1 h, and then heated to 250 o. C and held for 1 h. • After purification the CIGS nanoparticles were dispersed in toluene. Metal (Cu, In, Ga) complex Se + OLA/ODE 100 ℃ for 1 h 130 ℃ for 1 h 250 ℃ for 1 h Washed Dried

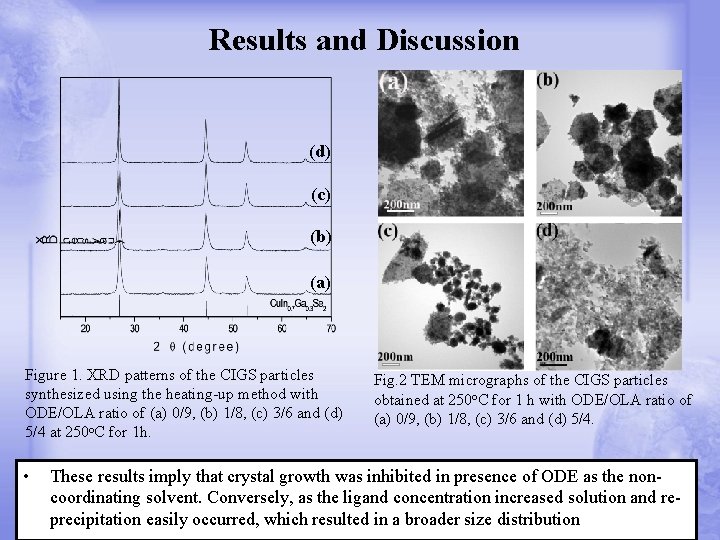
Results and Discussion (d) (c) (b) (a) Figure 1. XRD patterns of the CIGS particles synthesized using the heating-up method with ODE/OLA ratio of (a) 0/9, (b) 1/8, (c) 3/6 and (d) 5/4 at 250 o. C for 1 h. • Fig. 2 TEM micrographs of the CIGS particles obtained at 250 o. C for 1 h with ODE/OLA ratio of (a) 0/9, (b) 1/8, (c) 3/6 and (d) 5/4. These results imply that crystal growth was inhibited in presence of ODE as the noncoordinating solvent. Conversely, as the ligand concentration increased solution and reprecipitation easily occurred, which resulted in a broader size distribution

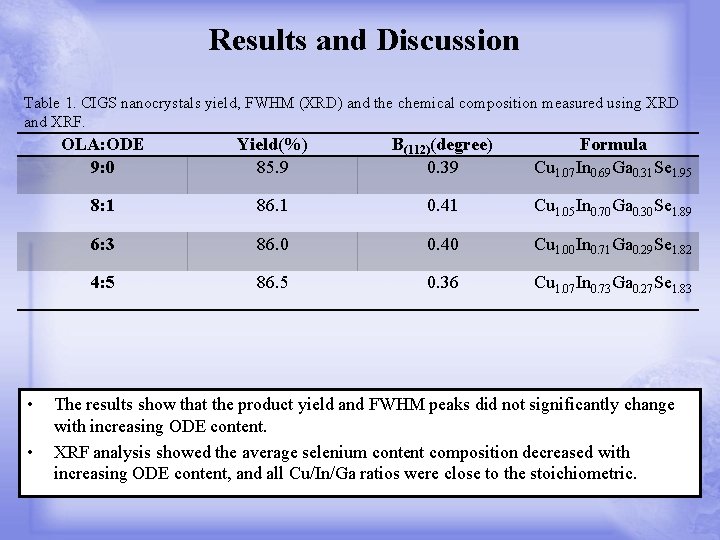
Results and Discussion Table 1. CIGS nanocrystals yield, FWHM (XRD) and the chemical composition measured using XRD and XRF. • • OLA: ODE 9: 0 Yield(%) 85. 9 Β(112)(degree) 0. 39 Formula Cu 1. 07 In 0. 69 Ga 0. 31 Se 1. 95 8: 1 86. 1 0. 41 Cu 1. 05 In 0. 70 Ga 0. 30 Se 1. 89 6: 3 86. 0 0. 40 Cu 1. 00 In 0. 71 Ga 0. 29 Se 1. 82 4: 5 86. 5 0. 36 Cu 1. 07 In 0. 73 Ga 0. 27 Se 1. 83 The results show that the product yield and FWHM peaks did not significantly change with increasing ODE content. XRF analysis showed the average selenium content composition decreased with increasing ODE content, and all Cu/In/Ga ratios were close to the stoichiometric.

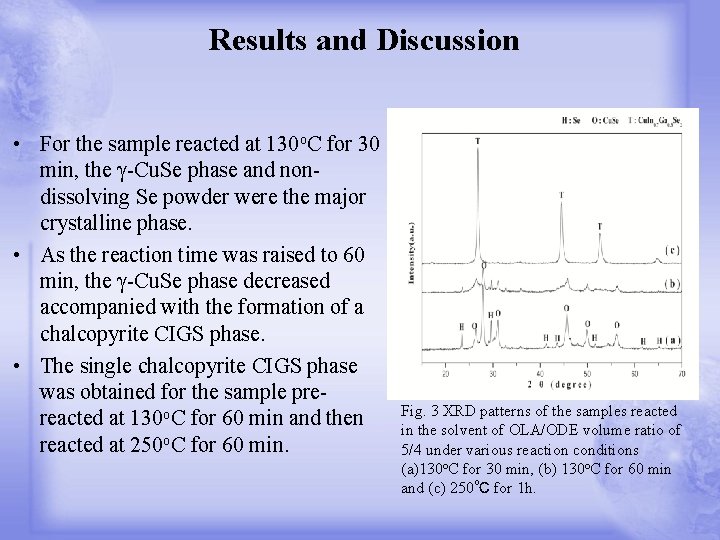
Results and Discussion • For the sample reacted at 130 o. C for 30 min, the γ-Cu. Se phase and nondissolving Se powder were the major crystalline phase. • As the reaction time was raised to 60 min, the γ-Cu. Se phase decreased accompanied with the formation of a chalcopyrite CIGS phase. • The single chalcopyrite CIGS phase was obtained for the sample prereacted at 130 o. C for 60 min and then reacted at 250 o. C for 60 min. Fig. 3 XRD patterns of the samples reacted in the solvent of OLA/ODE volume ratio of 5/4 under various reaction conditions (a)130 o. C for 30 min, (b) 130 o. C for 60 min and (c) 250℃ for 1 h.

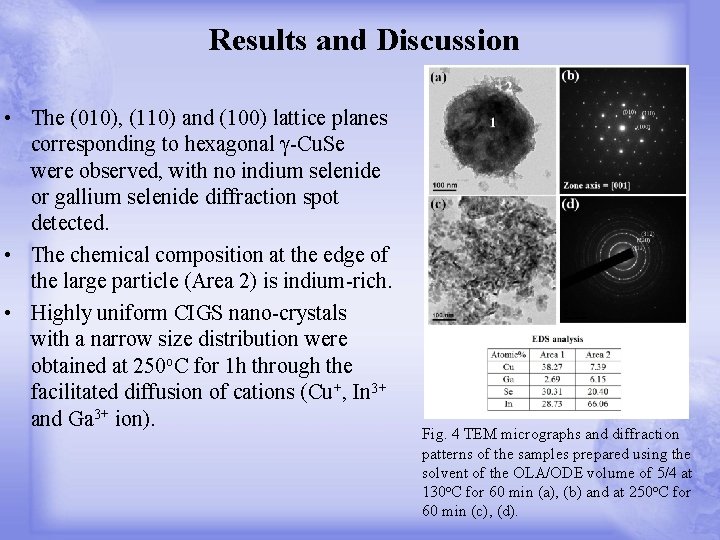
Results and Discussion • The (010), (110) and (100) lattice planes corresponding to hexagonal γ-Cu. Se were observed, with no indium selenide or gallium selenide diffraction spot detected. • The chemical composition at the edge of the large particle (Area 2) is indium-rich. • Highly uniform CIGS nano-crystals with a narrow size distribution were obtained at 250 o. C for 1 h through the facilitated diffusion of cations (Cu+, In 3+ and Ga 3+ ion). Fig. 4 TEM micrographs and diffraction patterns of the samples prepared using the solvent of the OLA/ODE volume of 5/4 at 130 o. C for 60 min (a), (b) and at 250 o. C for 60 min (c), (d).

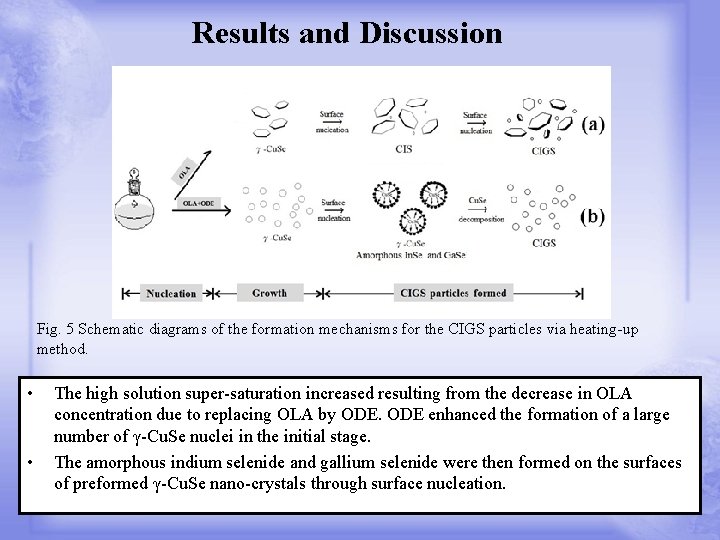
Results and Discussion Fig. 5 Schematic diagrams of the formation mechanisms for the CIGS particles via heating-up method. • • The high solution super-saturation increased resulting from the decrease in OLA concentration due to replacing OLA by ODE enhanced the formation of a large number of γ-Cu. Se nuclei in the initial stage. The amorphous indium selenide and gallium selenide were then formed on the surfaces of preformed γ-Cu. Se nano-crystals through surface nucleation.

Conclusions ① Cu. In 0. 7 Ga 0. 3 Se 2(CIGS) nano-crystals were successfully synthesized via a heating-up process in this study. The super-saturation in the solution can be adjusted by changing OLA/ODE ratio. ② As the superstation increased by increasing the ODE addition, Cu. Se nanocrystals appeared first, followed by amorphous indium selenide and gallium selenide nano-crystals formed on the surfaces of Cu. Se nanocrystallites through surface nucleation at the lower temperature and shorter reaction time. ③ As the reaction temperature was raised to 250 o. C the amorphous indium selenide and gallium selenide nano-crystals reacted with Cu. Se crystalline to form thermodynamically stable chalcopyrite CIGS nano-crystallites.

Thanks For Your Attention
 Cheng kung fu panda
Cheng kung fu panda Panyengkuyung tegese
Panyengkuyung tegese Ano ang layunin ng bugtong
Ano ang layunin ng bugtong Department of information engineering university of padova
Department of information engineering university of padova Information engineering padova
Information engineering padova University of sargodha engineering department
University of sargodha engineering department National taiwan university civil engineering
National taiwan university civil engineering Resource allocation vs resource leveling
Resource allocation vs resource leveling Contoh resource loading
Contoh resource loading Swot analysis for human resource department
Swot analysis for human resource department Swot analysis for human resource department
Swot analysis for human resource department What is the meaning of “national resource security”?
What is the meaning of “national resource security”? National charter school resource center
National charter school resource center























