NANOTECHNOLOGY IN NANOMEDICINE CASE STUDY Ineke Malsch Malsch








- Slides: 12

NANOTECHNOLOGY IN NANOMEDICINE CASE STUDY Ineke Malsch, Malsch Techno. Valuation Nano 2 All Webinar – October 2017 THE NANO 2 ALL PROJECT HAS RECEIVED FUNDING FROM THE EUROPEAN UNION’S HORIZON 2020 RESEARCH AND INNOVATION PROGRAMME, UNDER THE GRANT AGREEMENT NUMBER 685931. THIS PUBLICATION REFLECTS ONLY THE AUTHOR’S VIEW AND THE COMMISSION IS NOT RESPONSIBLE FOR ANY USE THAT MAY BE MADE OF THE INFORMATION IT CONTAINS.

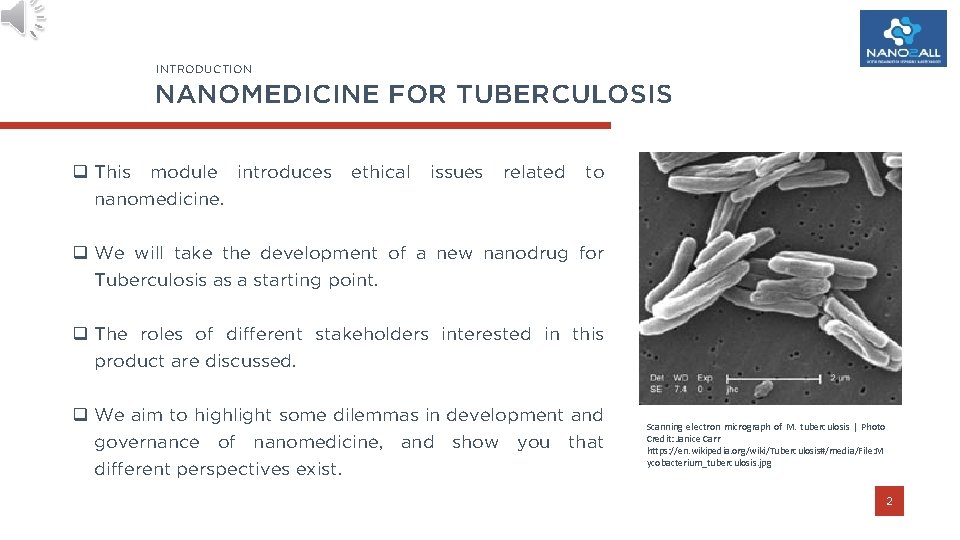
INTRODUCTION NANOMEDICINE FOR TUBERCULOSIS q This module introduces nanomedicine. ethical issues related to q We will take the development of a new nanodrug for Tuberculosis as a starting point. q The roles of different stakeholders interested in this product are discussed. q We aim to highlight some dilemmas in development and governance of nanomedicine, and show you that different perspectives exist. Scanning electron micrograph of M. tuberculosis | Photo Credit: Janice Carr https: //en. wikipedia. org/wiki/Tuberculosis#/media/File: M ycobacterium_tuberculosis. jpg 2

02. NANOMEDICINE FOR TUBERCULOSIS CASE STUDY DESCRIPTION In 2015: 10. 4 million Tuberculosis patients 1. 8 million deaths (>95% in low- and middleincome countries)* * Source: World Health Organisation website, 2017 Microscopy of tuberculous epididymitis. H&E stain | Department of Pathology, Calicut Medical college - Government Medical College, Kozhikode https: //en. wikipedia. org/wiki/Tuberculosis#/media/File: Tu berculous_epididymitis_Low_Power. jpg Therapies take over 6 months, causing severe side-effect. Patients are quarantined to ensure their therapy-loyalty. 3

02. NANOMEDICINE FOR TUBERCULOSIS CASE STUDY DESCRIPTION In South Africa, the Council for Scientific and Industrial Research (CSIR) was developing Rifanano, targeted nano drug delivery system of TB-drugs. This reduces drug intake from daily to 1 x/week, possibly only 2 months (from 6) and causes much less side-effects. Patients can probably stay home. Before the nanodrugs can be given to patients, their safety and efficacy must be demonstrated (by law). This takes many years. Trust Saidi conducted a study on stakeholder perceptions of the new drug and interviewed scientists, policy makers, patients, nurses and home care givers about their assessment of its risks and benefits before the product was available. Source: Travelling Nanotechnologies, Ph. D Thesis University Maastricht, 2016 4

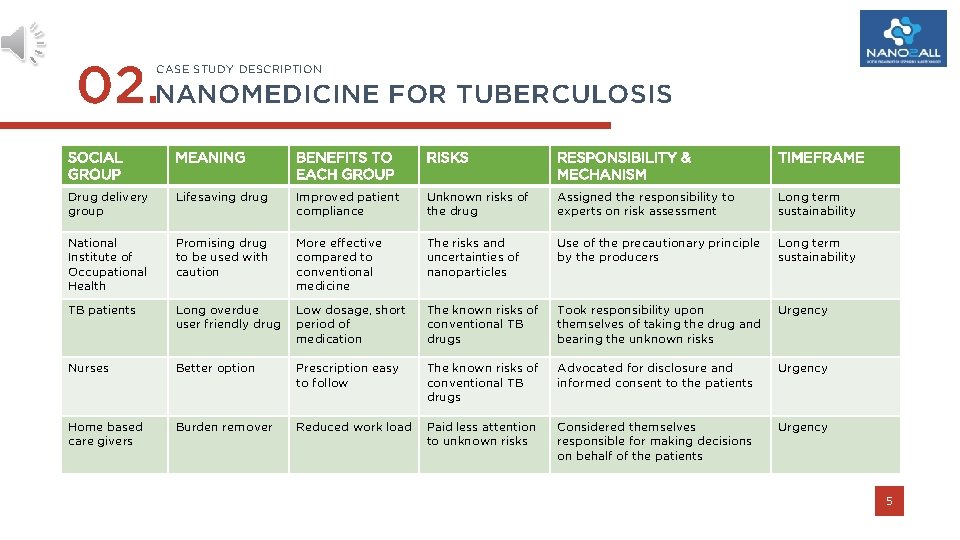
02. NANOMEDICINE FOR TUBERCULOSIS CASE STUDY DESCRIPTION SOCIAL GROUP MEANING BENEFITS TO EACH GROUP RISKS RESPONSIBILITY & MECHANISM TIMEFRAME Drug delivery group Lifesaving drug Improved patient compliance Unknown risks of the drug Assigned the responsibility to experts on risk assessment Long term sustainability National Institute of Occupational Health Promising drug to be used with caution More effective compared to conventional medicine The risks and uncertainties of nanoparticles Use of the precautionary principle by the producers Long term sustainability TB patients Long overdue user friendly drug Low dosage, short period of medication The known risks of conventional TB drugs Took responsibility upon themselves of taking the drug and bearing the unknown risks Urgency Nurses Better option Prescription easy to follow The known risks of conventional TB drugs Advocated for disclosure and informed consent to the patients Urgency Home based care givers Burden remover Reduced work load Paid less attention to unknown risks Considered themselves responsible for making decisions on behalf of the patients Urgency 5

Image: Argonne National Laboratory FLICKR https: //www. flickr. com/photos/argonne/ 14730293770 02. NANOMEDICINE FOR TUBERCULOSIS CASE STUDY DESCRIPTION q Traditionally, patients, nurses, home care givers and other citizens are not involved in (pre-clinical) drug development but do suffer side effects of existing drugs q Scientists, risk assessors and authorities abide by pre-existing laws and focus on the unknown risks of the new drug, not taking into account the existing risks of already available drugs for Tuberculosis 6

Image: Argonne National Laboratory FLICKR https: //www. flickr. com/photos/argonne/ 14730293770 02. NANOMEDICINE FOR TUBERCULOSIS CASE STUDY DESCRIPTION q Information on Rifanano was only available to the scientists and safety assessors in the National Institute of Occupational Health. What could more responsible nanodrug development look like? q Trust Saidi acted as a bridge between both communities 7

03. CITIZENS QUESTIONS FOR STAKEHOLDERS BE CURIOUS • Only after learning about the new nanodrug could patients, nurses and care givers form an opinion about it. REFLECT ON DILEMMAS • How can the risks and benefits of the nanodrug and existing therapy be balanced? • Which underlying values are at stake? Note the different assignments of responsibility. TARGET THE RIGHT LEVEL • Market acceptance of drugs is well-regulated. Any change calls for wide public debate and political decision making. 8

03. SCIENTISTS QUESTIONS FOR STAKEHOLDERS The natural scientists already adhere by research ethics principles Value-Sensitive Design is addressed through nanoencapsulation. It could be improved if the patients, nurses and family would have been engaged before starting the research The social scientist could engage in science for policy by communicating their findings to policy makers Natural and social scientists could engage in public dialogue exploring if the current regulations are adequate for nanodrugs Image: Novartis AG FLICKR https: //www. flickr. com/photos /51868421@N 04/26118650305 / 9

03. OTHERS QUESTIONS FOR STAKEHOLDERS Policy makers could reflect on the appropriateness of current regulations in balancing the need for short term solutions and long term uncertain risk Industry, media and CSOs could consider how to contribute to the public dialogue 10

04. RESPONSIBLE DIALOGUE NANOCURE FOR CONCLUSION TUBERCULOSIS q The complexity of the issues surrounding the development of a new nanomedicine for Tuberculosis merits careful scrutiny of the interests of different stakeholders q The issues are a mix of technological, ethical, economic and political aspects q A universal law such as the one regulating market access of pharmaceuticals may be too rigid - no legal room exists to let patients decide if they wish to accept the unknown risks of the new drug q Flexible framework regulations offering room for social experiments may be preferred to formal universal laws 11

THANK YOU