MO Theory H 2 and H 2 solns









- Slides: 40

MO Theory H 2+ and H 2 solns

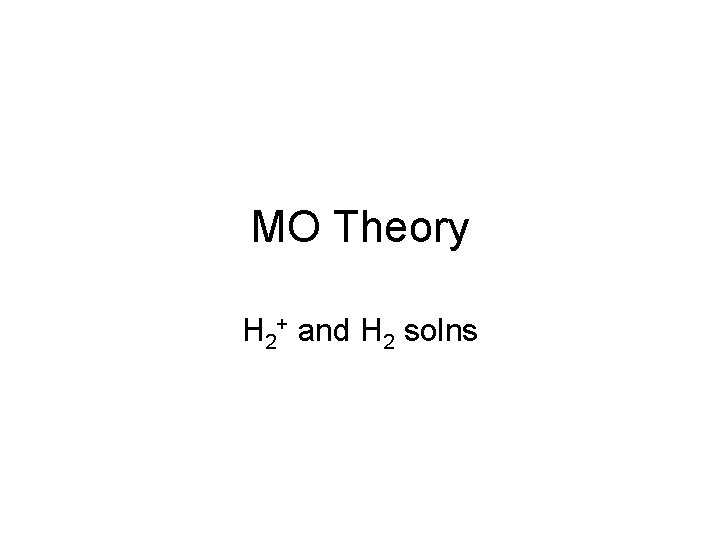
Solutions to Hydrogen Molecule Ion Y 2, E 2 = -10. 16 e. V (for H 2 ) Y 1, E 1 = 1. 37 e. V (for H 2)

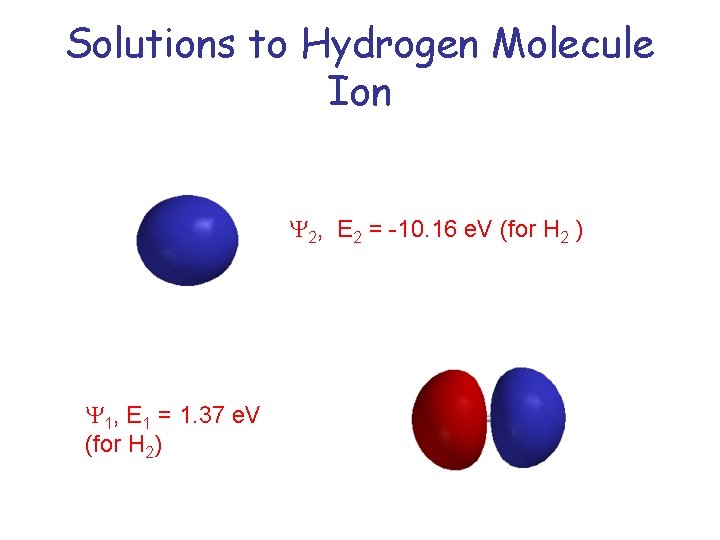
Solutions to Hydrogen Molecule MOs created from combinations of p-orbitals px. A + px. B, py. A + py. B px. A - px. B, py. A - py. B pz. A - pz. B pz. A + pz. B

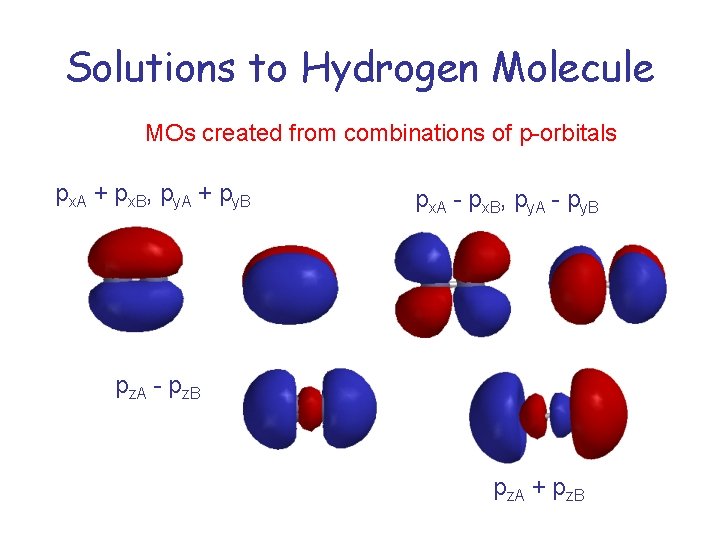
Solutions to Hydrogen Molecule px+ px OR py + py px - px OR py - py pz - pz pz + pz

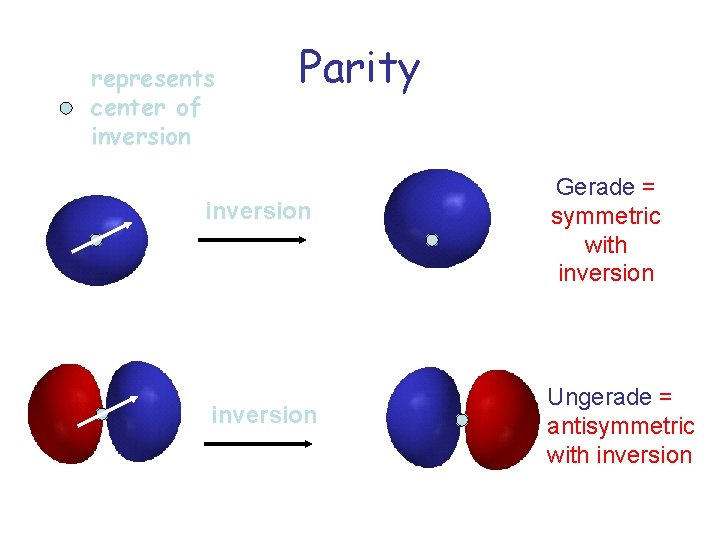
represents center of inversion Parity inversion Gerade = symmetric with inversion Ungerade = antisymmetric with inversion

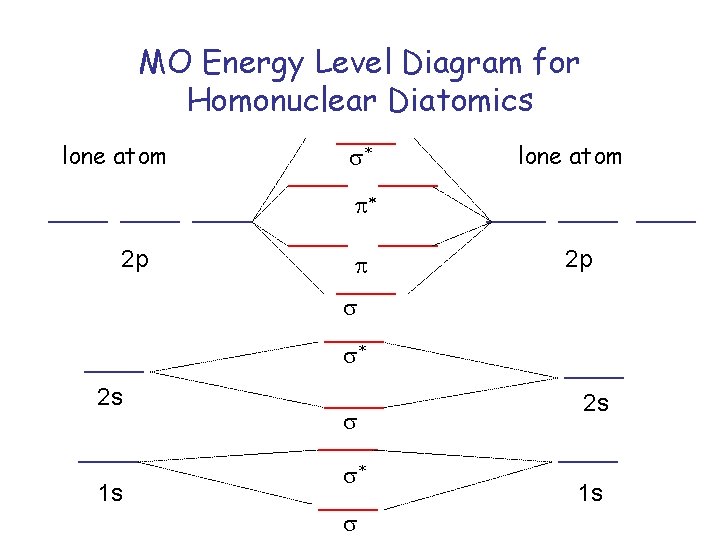
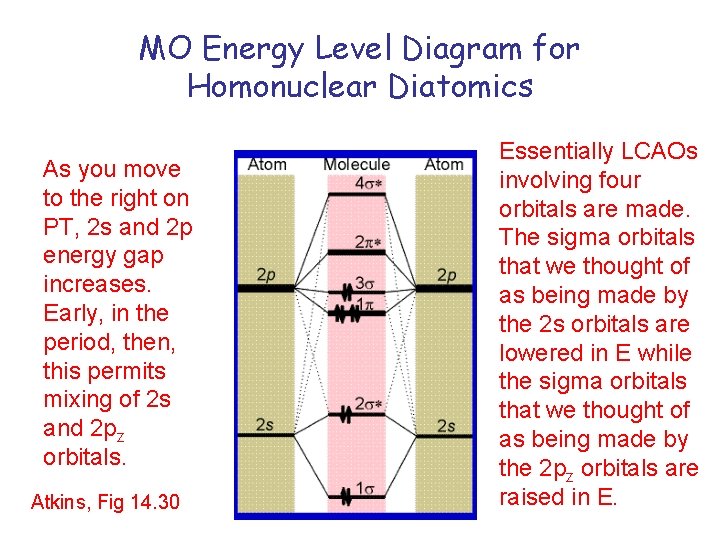
MO Energy Level Diagram for Homonuclear Diatomics lone atom s* lone atom p* 2 p p 2 p s s* 2 s 1 s s s* s 2 s 1 s

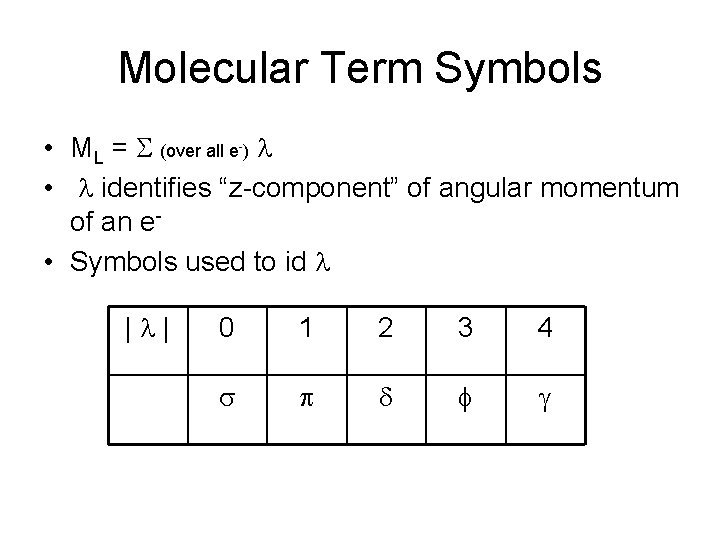
Molecular Term Symbols • ML = S (over all e-) l • l identifies “z-component” of angular momentum of an e • Symbols used to id l |l| 0 1 2 3 4 s p d f g

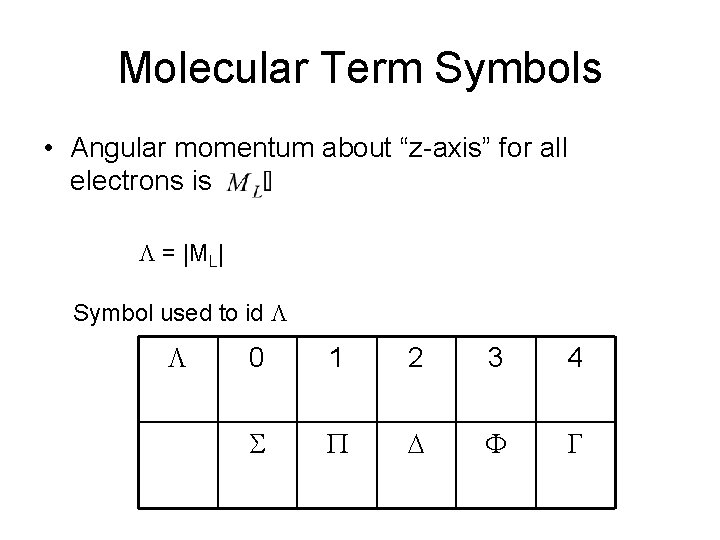
Molecular Term Symbols • Angular momentum about “z-axis” for all electrons is L = |ML| Symbol used to id L L 0 1 2 3 4 S P D F G

Molecular Term Symbols • Symbol is 2 S + 1 L g/u • 2 S + 1 is multiplicity as already used for atomic term symbols • g or u identifies overall parity – To determine overall parity, make use of multiplication of symmetric and antisymmetric functions • If the term is a S term, a right superscript of + or – is added to indicate whether the wavefunction is symmetric or antisymmetric with respect to reflection through a plane containing the two nuclei

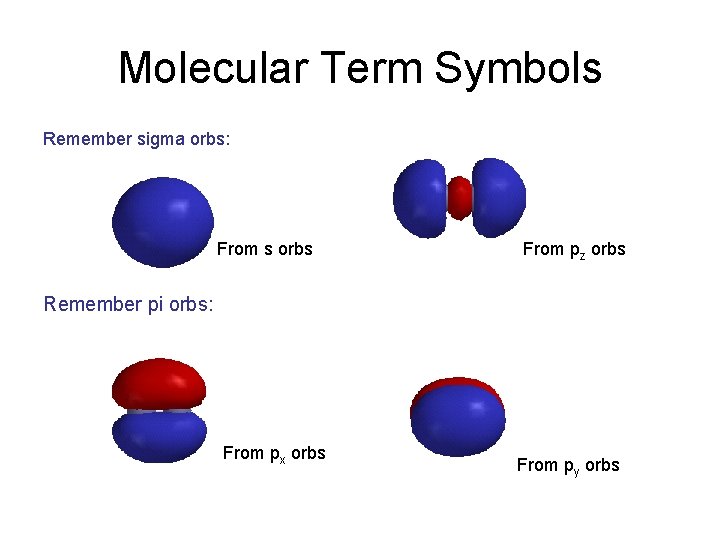
Molecular Term Symbols Remember sigma orbs: From s orbs From pz orbs Remember pi orbs: From px orbs From py orbs

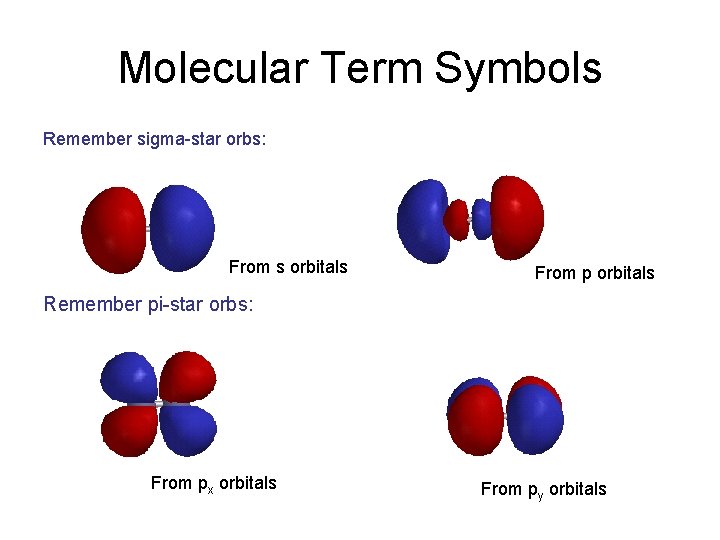
Molecular Term Symbols Remember sigma-star orbs: From s orbitals From p orbitals Remember pi-star orbs: From px orbitals From py orbitals

Spectroscopy – Selection Rules DL = 0, +1, -1 DS = 0 note S = Ms DS = 0 note W refers to spin-orbit coupling and W=L+S DW = 0, +1, -1

Molecular Term Symbols • Molecular Orbitals not always so “clearcut” • Remember how orbitals change energy as go across PT – Can affect MO energy pattern too

MO Energy Level Diagram for Homonuclear Diatomics As you move to the right on PT, 2 s and 2 p energy gap increases. Early, in the period, then, this permits mixing of 2 s and 2 pz orbitals. Atkins, Fig 14. 30 Essentially LCAOs involving four orbitals are made. The sigma orbitals that we thought of as being made by the 2 s orbitals are lowered in E while the sigma orbitals that we thought of as being made by the 2 pz orbitals are raised in E.

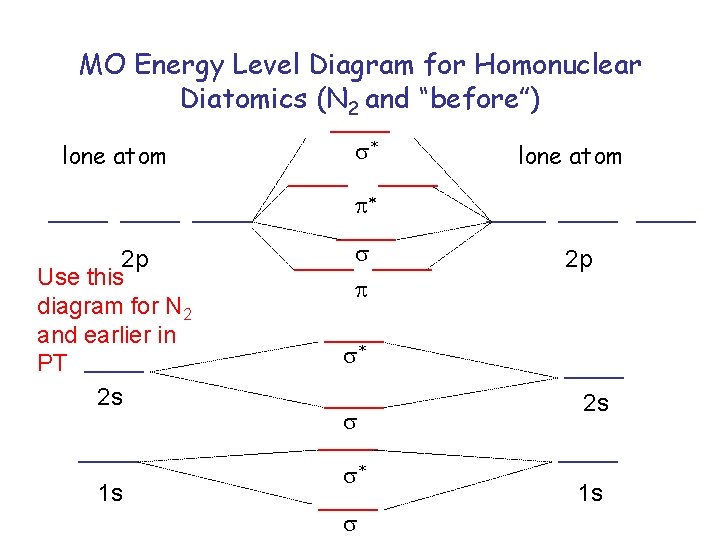
MO Energy Level Diagram for Homonuclear Diatomics (N 2 and “before”) lone atom s* lone atom p* 2 p Use this diagram for N 2 and earlier in PT 2 s 1 s s p 2 p s* s 2 s 1 s

Taking a look at heteronuclear diatomic molecules

Taking a look at heteronuclear diatomic molecules

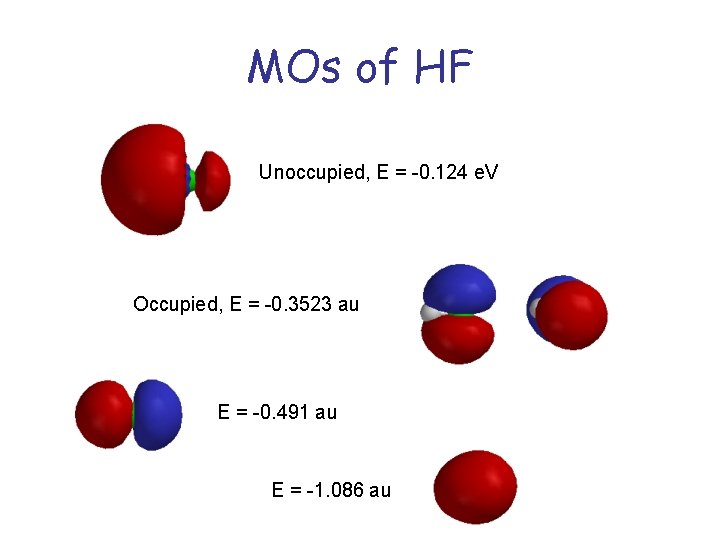
MOs of HF Unoccupied, E = -0. 124 e. V Occupied, E = -0. 3523 au E = -0. 491 au E = -1. 086 au

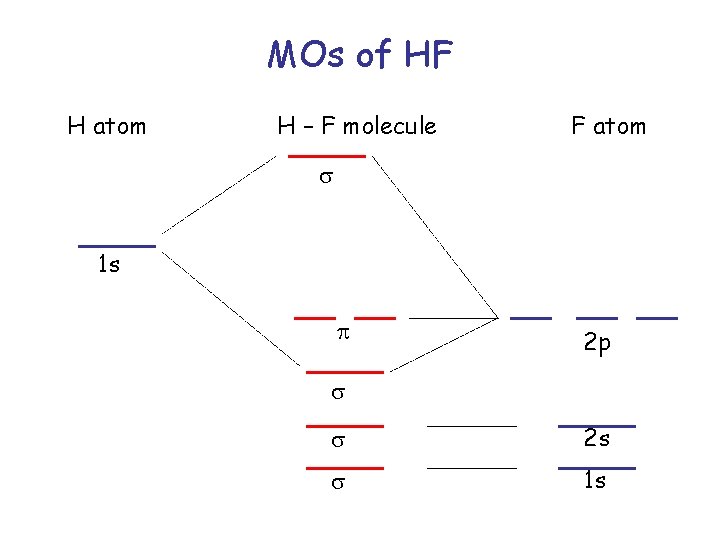
MOs of HF H atom H – F molecule F atom s 1 s p 2 p s s 2 s s 1 s

Computational Chemistry • Considering complexity of the calculations we’ve been doing, certainly, using computers to do these calcs should be useful Computational Chemistry • For polyatomic molecules can make LCAOs y. MO = S ciyi - Yi constitute basis set (computational forms of atomic orbitals) – Use variation theory to find ci – To find structure of molecule, must move nuclei and find MOs find structure with lowest overall energy

Computational Chemistry • May “solve” for MOs using ab initio or semiempirical methods – Semi-empirical methods: empirical parameters substituted for some “integrals” to save time in calculations – Ab initio methods: supposedly make no assumptions • NOTE: computational chemistry may determine Energy and some other properties without using quantum chemistry – Such calculations are referred to as molecular mechanics calculations

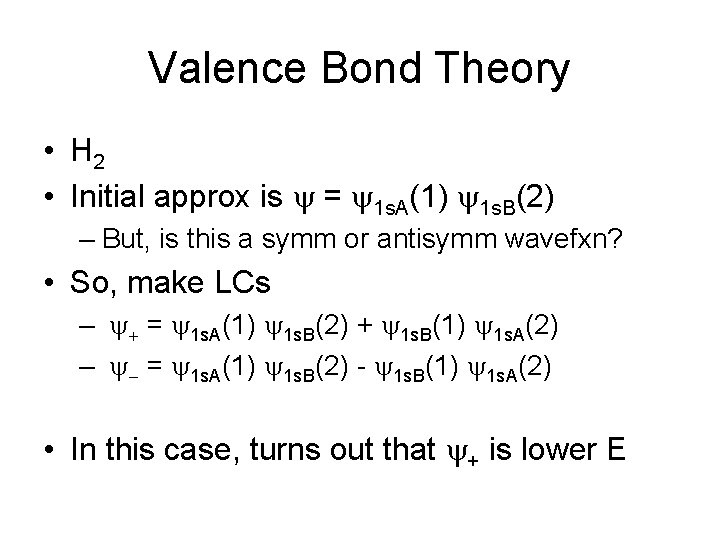
Valence Bond Theory • H 2 • Initial approx is y = y 1 s. A(1) y 1 s. B(2) – But, is this a symm or antisymm wavefxn? • So, make LCs – y+ = y 1 s. A(1) y 1 s. B(2) + y 1 s. B(1) y 1 s. A(2) – y- = y 1 s. A(1) y 1 s. B(2) - y 1 s. B(1) y 1 s. A(2) • In this case, turns out that y+ is lower E

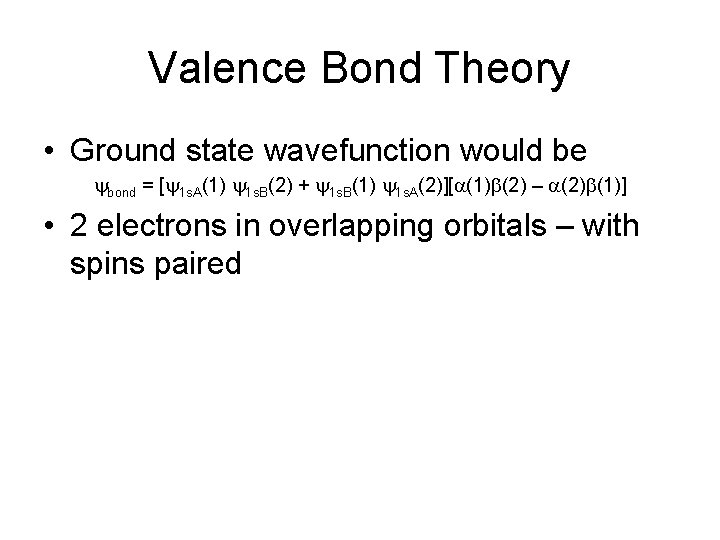
Valence Bond Theory • Ground state wavefunction would be ybond = [y 1 s. A(1) y 1 s. B(2) + y 1 s. B(1) y 1 s. A(2)][a(1)b(2) – a(2)b(1)] • 2 electrons in overlapping orbitals – with spins paired

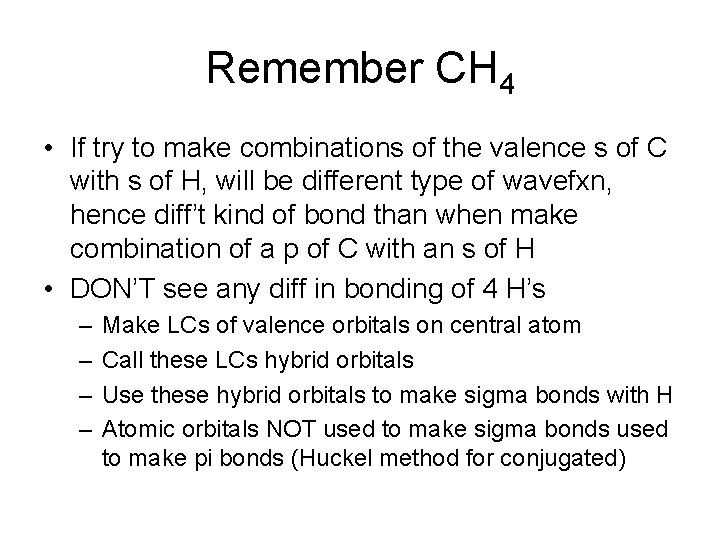
Remember CH 4 • If try to make combinations of the valence s of C with s of H, will be different type of wavefxn, hence diff’t kind of bond than when make combination of a p of C with an s of H • DON’T see any diff in bonding of 4 H’s – – Make LCs of valence orbitals on central atom Call these LCs hybrid orbitals Use these hybrid orbitals to make sigma bonds with H Atomic orbitals NOT used to make sigma bonds used to make pi bonds (Huckel method for conjugated)

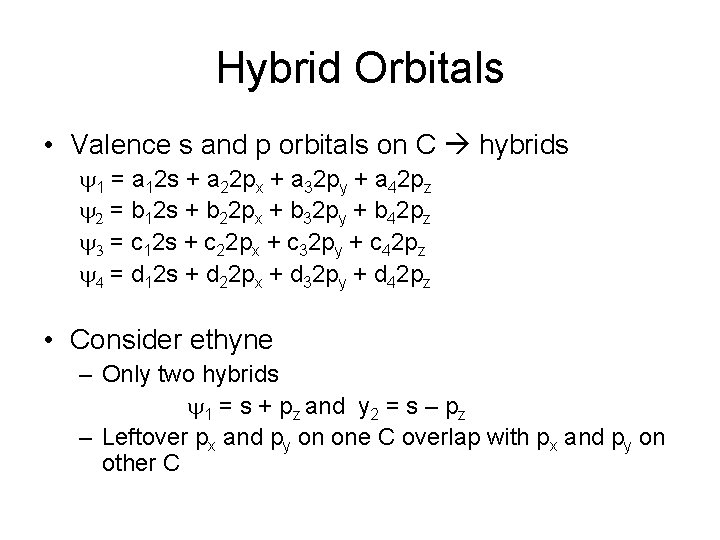
Hybrid Orbitals • Valence s and p orbitals on C hybrids y 1 = a 12 s + a 22 px + a 32 py + a 42 pz y 2 = b 12 s + b 22 px + b 32 py + b 42 pz y 3 = c 12 s + c 22 px + c 32 py + c 42 pz y 4 = d 12 s + d 22 px + d 32 py + d 42 pz • Consider ethyne – Only two hybrids y 1 = s + pz and y 2 = s – pz – Leftover px and py on one C overlap with px and py on other C

Simplification to MO Approach Huckel Approach

Symmetry of Molecules

Determining Point Groups

Special Group? No No C 1 i No sh No Yes Cn Yes Cs Ci No n sv No Yes No Cn sh Cnv Yes C∞v , D∞h , Td , Oh , Ih , Th Yes No S 2 n or S 2 n and i only, collinear with highest order Cn Sn Yes n. C 2 perpendicular to Cn Yes Cnh Dn No No n sd Yes sh Yes Dnh Dnd

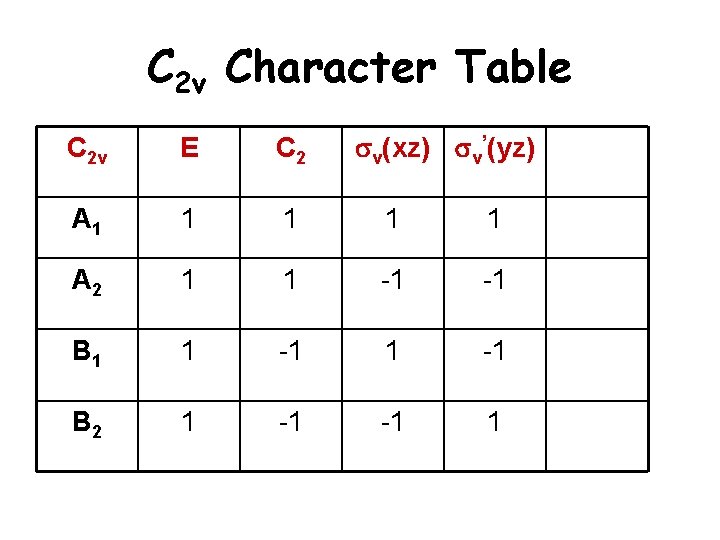
C 2 v Character Table sv(xz) sv’(yz) C 2 v E C 2 A 1 1 1 A 2 1 1 -1 -1 B 1 1 -1 B 2 1 -1 -1 1

Now go practice!!!

Applying Symmetry to MOs Water

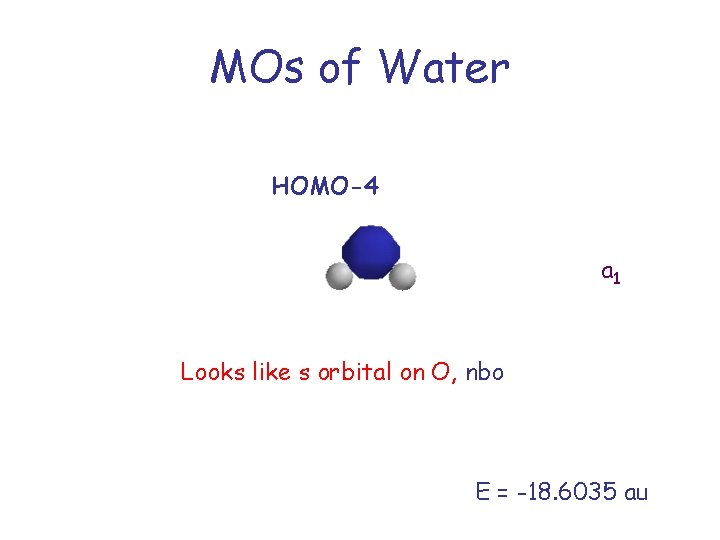
MOs of Water HOMO-4 a 1 Looks like s orbital on O, nbo E = -18. 6035 au

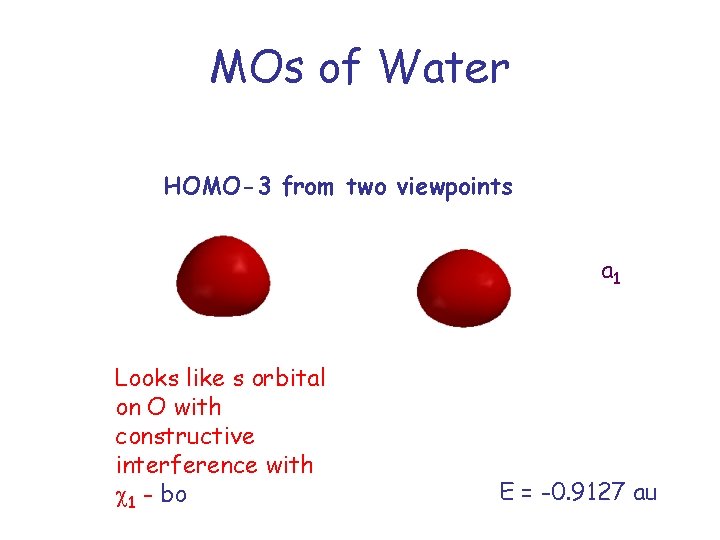
MOs of Water HOMO-3 from two viewpoints a 1 Looks like s orbital on O with constructive interference with c 1 - bo E = -0. 9127 au

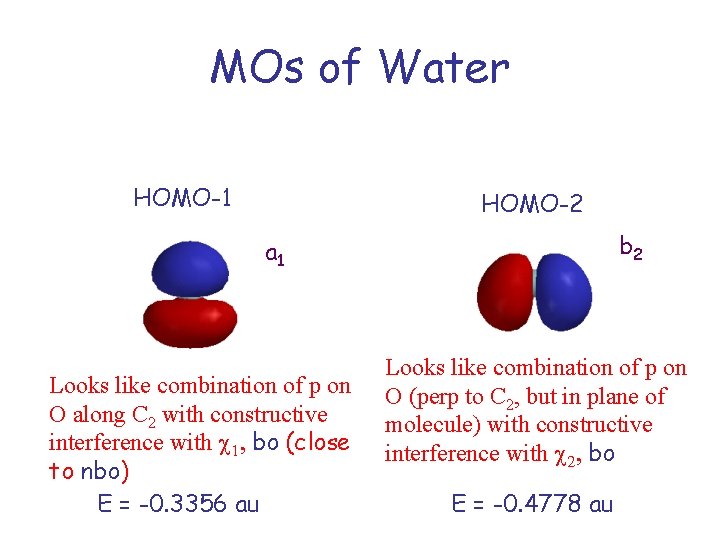
MOs of Water HOMO-1 HOMO-2 b 2 a 1 Looks like combination of p on O along C 2 with constructive interference with c 1, bo (close to nbo) E = -0. 3356 au Looks like combination of p on O (perp to C 2, but in plane of molecule) with constructive interference with c 2, bo E = -0. 4778 au

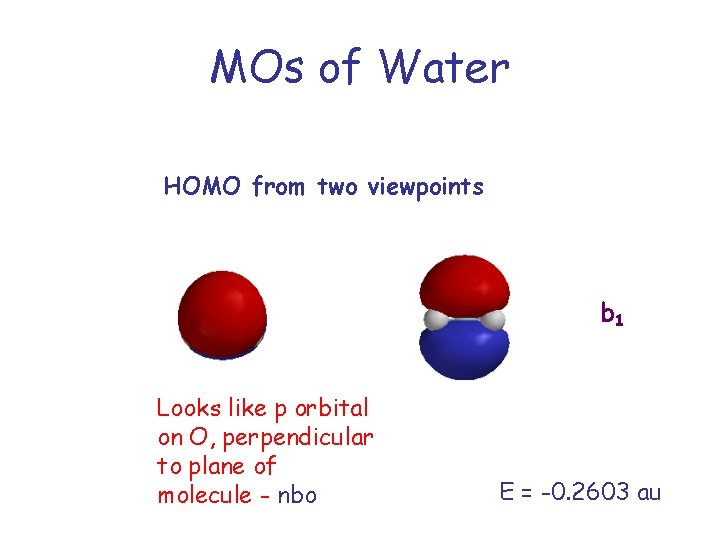
MOs of Water HOMO from two viewpoints b 1 Looks like p orbital on O, perpendicular to plane of molecule - nbo E = -0. 2603 au

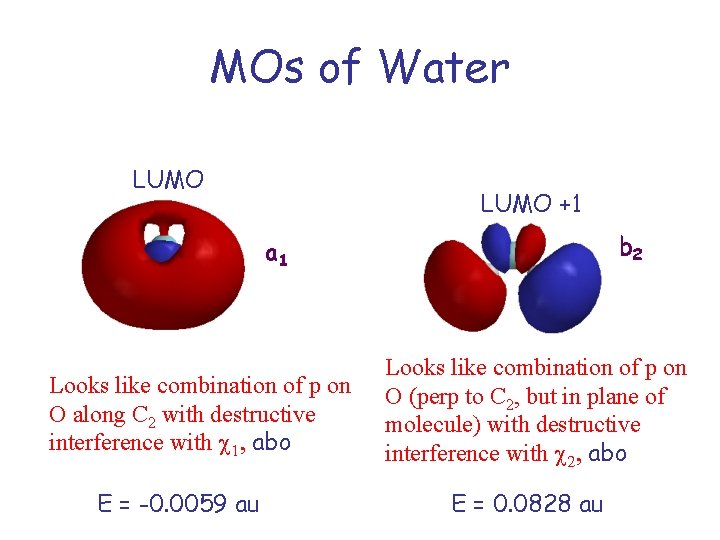
MOs of Water LUMO +1 b 2 a 1 Looks like combination of p on O along C 2 with destructive interference with c 1, abo E = -0. 0059 au Looks like combination of p on O (perp to C 2, but in plane of molecule) with destructive interference with c 2, abo E = 0. 0828 au

Filling Pattern for Water 2 b 2 (abo) 4 a 1 (abo) 1 b 1 (nbo) 3 a 1 (bo/nbo) 1 b 2 (bo) 2 a 1 (bo) 1 a 1 (nbo)

Molecular Spectroscopy • Molecule has a number of motions – Translational, vibrational, rotational, electronic • Sum them to get total energy of molecule • Changes may occur in any of these modes through absorption or emission of energy – Vibrational: IR – Rotational: Microwave – Electronic: UV-Vis CHP 16, 17, 18 of text

Statistical Mechanics • Quantum gives you possible energy levels (states) – In a real sample, not all molecules in the same energy level • With statistics and total energy, can predict (on average) how many molecules in each state – Dynamic Equilibrium – Role of Temperature • Can predict macroscopic properties/behavior – Heat capacity, pressure, etc. CHP 19, 20 of text