Minerals WHAT IS A MINERAL HOW DO MINERALS









- Slides: 15

Minerals WHAT IS A MINERAL? HOW DO MINERALS FORM? HOW CAN MINERALS BE IDENTIFIED?

Key Terms v Mineral v Crystal form v Lattice v Density v Luster v Inorganic v Streak v Chemical formula v Hardness v Cleavage v Fracture Minerals are the building blocks of rocks, and therefore of the earth itself. To be a Mineral, Something Must. . .

Naturally Occurring Solid Inorganic Orderly Crystal Grid or Lattice Have A Definite Chemical Formula Naturally occurring means the substance cannot be human-created. For example: Ice found in snowflakes or glaciers are minerals Ice in your kitchen freezer is not made naturally and is not a mineral. Liquids and gases can become minerals only if they change to a solid.

Solid Naturally Occurring Always solid state of matter Solid Definite volume and shape Particles are packed together very tightly Orderly Crystal Grid or Lattice Cannot move compared to a Have A Definite Chemical liquid or gas Formula Inorganic

Inorganic or Organic Naturally Occurring Solid Inorganic means carbon that comes from living things is not present. Minerals are not formed Orderly Crystal Grid or Lattice from organic materials such Have A Definite Chemical as plant or animal remains. Formula

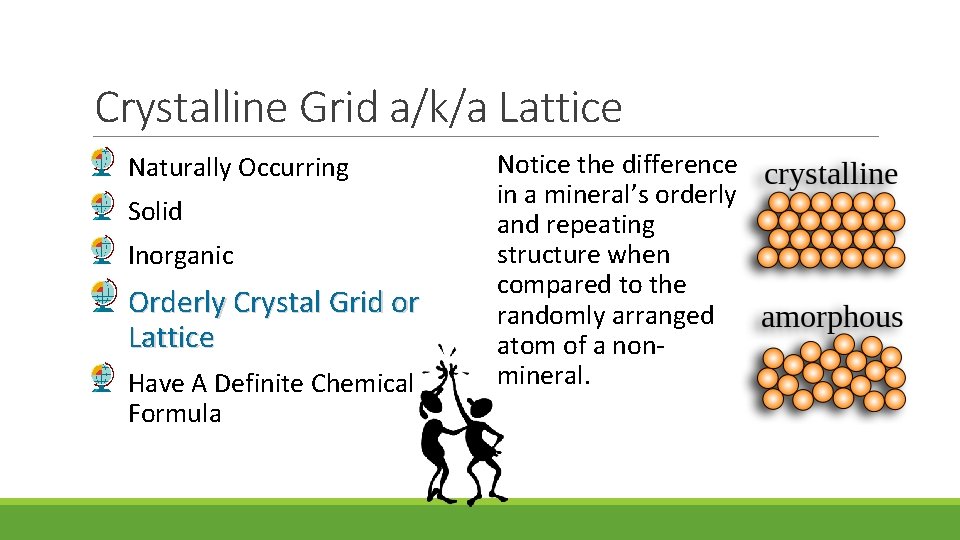
Crystalline Grid or Lattice Naturally Occurring Solid Inorganic Orderly Crystal Grid or Lattice Have A Definite Chemical Formula The atoms in minerals are arranged in an orderly pattern that repeats itself throughout the substance This structure, because of the chemical formula, is called crystalline.

Crystalline Grid a/k/a Lattice Naturally Occurring Solid Inorganic Orderly Crystal Grid or Lattice Have A Definite Chemical Formula Notice the difference in a mineral’s orderly and repeating structure when compared to the randomly arranged atom of a nonmineral.

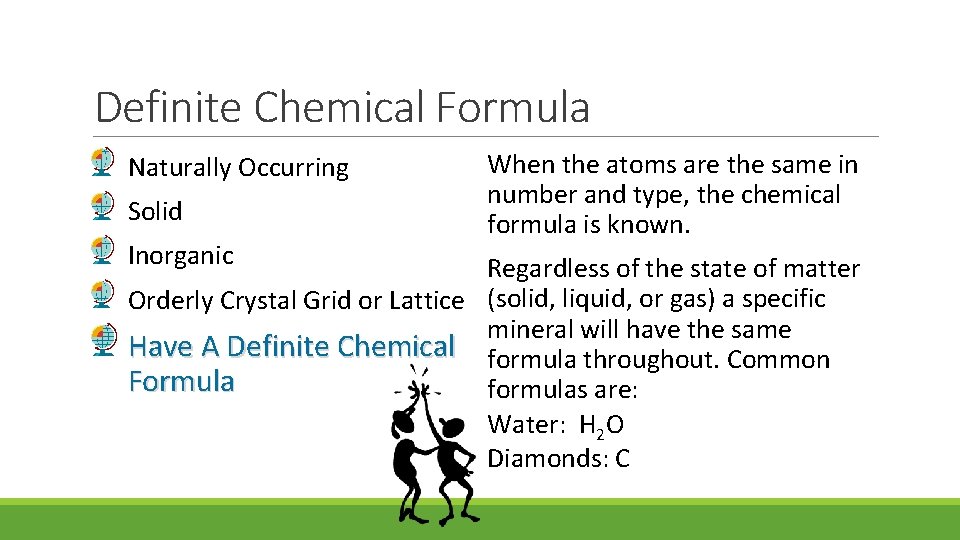
Definite Chemical Formula Naturally Occurring Solid Inorganic When the atoms are the same in number and type, the chemical formula is known. Regardless of the state of matter Orderly Crystal Grid or Lattice (solid, liquid, or gas) a specific mineral will have the same Have A Definite Chemical formula throughout. Common Formula formulas are: Water: H 2 O Diamonds: C

Minerals Form in Four Ways Crystallization from magma or other liquids Precipitation from solution Heat and Pressure Hydrothermal solutions Crystalline structure can occur when melted rock material cools into a solid. Water will crystalize when a lake freezes over and ice covers its surface.

Minerals Form in Four Ways Crystallization from magma or other liquids Precipitation from solution Heat and Pressure Hydrothermal solutions Minerals dissolved in water (solution) can precipitate (fall to the bottom) when the concentration is high enough.

Minerals Form in Four Ways Crystallization from magma or other liquids Precipitation from solution Heat and Pressure Hydrothermal solutions Extreme heat and pressure in the Earth can cause minerals to change form – without actually melting them. Because of this, different chemical bonds can form that changes the crystal lattice.

Minerals Form in Four Ways Crystallization from magma or other liquids Precipitation from solution Heat and Pressure Hydrothermal solutions Beneath the Earth’s surface water is super-heated so that it remains liquid instead of becoming steam. When this super-heated chemical solution contacts rocks and other minerals a new mineral is formed as a result other the chemical reaction.

Mineral Identification 1. Color – Natural appearance 2. Streak – Powdered form 3. Luster – Metallic or Non- Metallic

Mineral Identification 4. Hardness – Moh’s Hardness Scale 5. Crystal System – Six structures

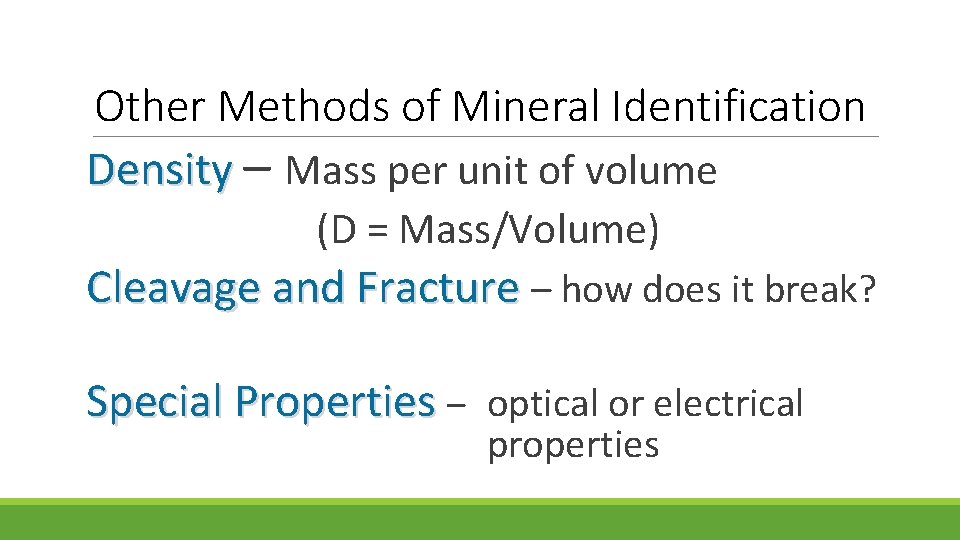
Other Methods of Mineral Identification Density – Mass per unit of volume (D = Mass/Volume) Cleavage and Fracture – how does it break? Special Properties – optical or electrical properties