Method 1 Molar Enthalpies of Reaction r Hm








- Slides: 15

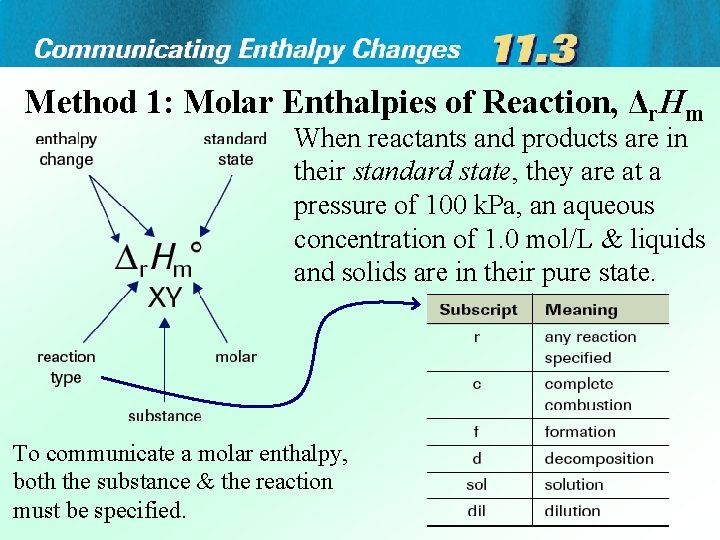
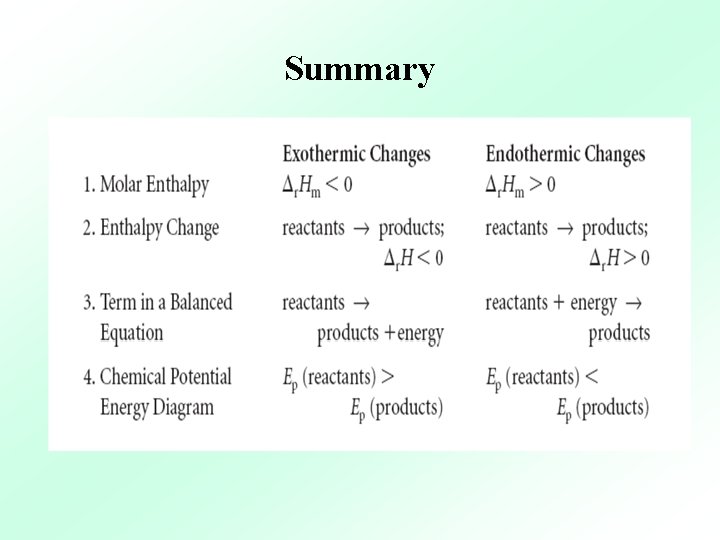
Method 1: Molar Enthalpies of Reaction, Δr. Hm When reactants and products are in their standard state, they are at a pressure of 100 k. Pa, an aqueous concentration of 1. 0 mol/L & liquids and solids are in their pure state. To communicate a molar enthalpy, both the substance & the reaction must be specified.

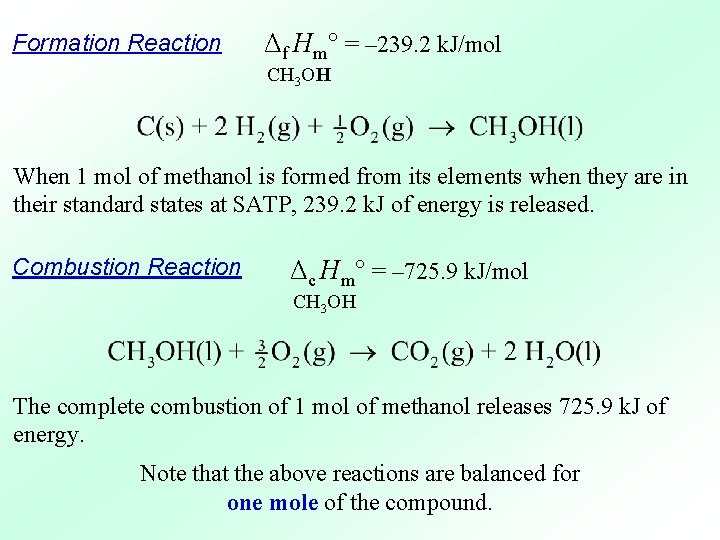
Formation Reaction Combustion Reaction Note that the above reactions are balanced for one mole of the compound.

Formation Reaction Δf Hm° = – 239. 2 k. J/mol CH 3 OH When 1 mol of methanol is formed from its elements when they are in their standard states at SATP, 239. 2 k. J of energy is released. Combustion Reaction Δc Hm° = – 725. 9 k. J/mol CH 3 OH The complete combustion of 1 mol of methanol releases 725. 9 k. J of energy. Note that the above reactions are balanced for one mole of the compound.

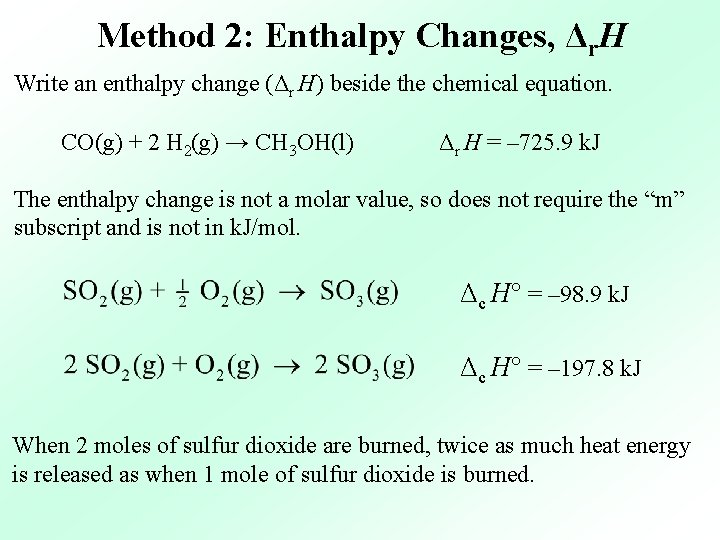
Method 2: Enthalpy Changes, Δr. H Write an enthalpy change (Δr H) beside the chemical equation. CO(g) + 2 H 2(g) → CH 3 OH(l) Δr H = – 725. 9 k. J The enthalpy change is not a molar value, so does not require the “m” subscript and is not in k. J/mol. Δc H° = – 98. 9 k. J Δc H° = – 197. 8 k. J When 2 moles of sulfur dioxide are burned, twice as much heat energy is released as when 1 mole of sulfur dioxide is burned.



Method 3: Energy Terms in Balanced Equations For endothermic reactions, the energy is listed along with the reactants + energy → products For exothermic reactions, the energy is listed along with the products. reactants → products + energy


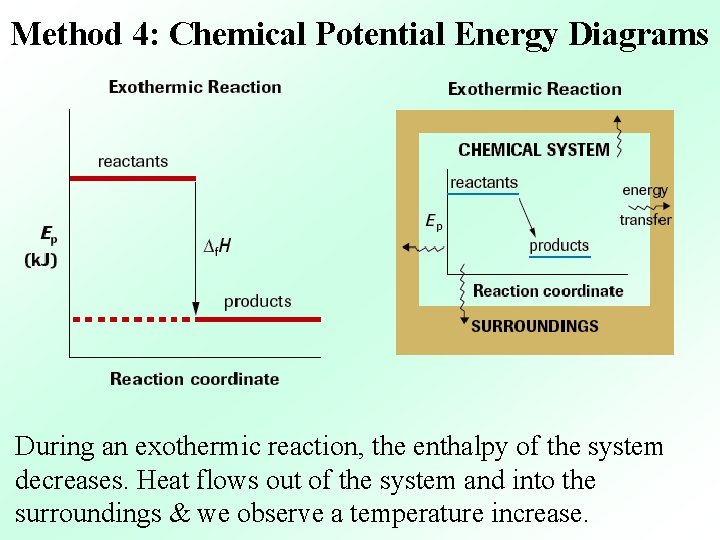
Method 4: Chemical Potential Energy Diagrams During an exothermic reaction, the enthalpy of the system decreases. Heat flows out of the system and into the surroundings & we observe a temperature increase.

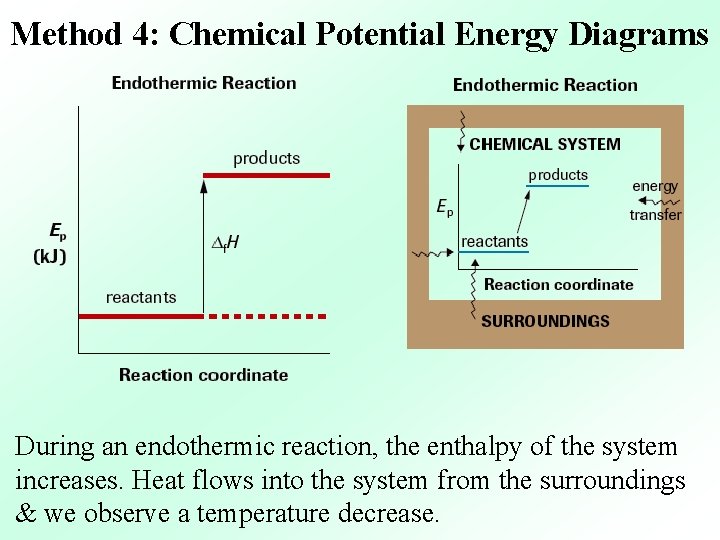
Method 4: Chemical Potential Energy Diagrams During an endothermic reaction, the enthalpy of the system increases. Heat flows into the system from the surroundings & we observe a temperature decrease.




Summary

ü Read pgs. 495 – 500 ü Section 11. 3 Questions #’s 1, 3, 4, 5 (p. 501)
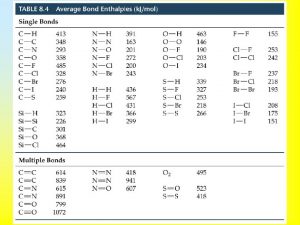
 Bond enthalpy reactants minus products
Bond enthalpy reactants minus products Limitations of average bond enthalpies
Limitations of average bond enthalpies Rate reaction equation
Rate reaction equation E1cb elimination reaction
E1cb elimination reaction Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Proton capture equation
Proton capture equation Introduction of symposium
Introduction of symposium Half reaction method
Half reaction method Half reaction method
Half reaction method Half reaction method
Half reaction method Principle of blood glucose estimation
Principle of blood glucose estimation Nh42so
Nh42so Molar formülü
Molar formülü Mass of phosphate
Mass of phosphate Volumen molar de ch4
Volumen molar de ch4 Absortividade molar
Absortividade molar