MEDIA PREPARATION AND STERILIZATION A MEDIUM IS STERILIZED
















- Slides: 32

MEDIA PREPARATION AND STERILIZATION

A MEDIUM IS STERILIZED (LIVING ORGANISMS REMOVED) BEFORE USAGE IN THE LAB. STERILIZATION METHODS INCLUDE; AUTOCLAVING, DRY-HEAT, FILTRATION, UV EXPOSURE AND ETHYLENE OXIDE. CULTURE: IS PART OF SPECIMEN GROWN IN CULTURE MEDIA: IS A MEDIUM (LIQUID OR SOLID) THAT CONTAINS NUTRIENTS TO GROW BACTERIA IN VITRO. BECAUSE SOMETIMES WE CANNOT IDENTIFY WITH MICROSCOPICAL EXAMINATION DIRECTLY, AND SOMETIMES WE DO CULTURE FOR ANTIBIOTIC SENSITIVITY TESTING.

MEDIUM IS A NUTRIENT BLEND USED TO SUPPORT MICROBIAL GROWTH. THERE ARE THREE PHYSICAL FORMS OF MEDIA, BROTH, SOLID, AND SEMISOLID MEDIA ARE MORE VERSATILE IN THEIR USAGE. * PROMOTE SURFACE GROWTH * USED TO ISOLATE PURE CULTURES * IDEAL FOR CULTURE STORAGE * HELPFUL IN THE OBSERVATION OF BIOCHEMICAL REACTIONS * USED TO MAKE SLANTS, DEEPS, AND PLATES (NAMED BY MEDIUM)

PROPERTIES OF MEDIA: Support the growth of the bacteria. Should be nutritive (contains the required amount of nutrients). Suitable p. H (neutral to slightly alkaline 7. 3 -7. 4). Suitable temperature, and suitable atmosphere. (Bacteria grow at 370 C) Note: media are sterilized by autoclaving at 1210 C and 1. 02 atmosphere (15 p. s. i. ) for 15 -20 minutes. With the autoclave, all bacteria, fungi, viruses, and spores are destroyed. Some media can’t be sterilized by autoclaving because they contain eggs or carbohydrates.

FORMS OF CULTURE Solid (agar): Is Broth plus agar (seaweed). Are prepared by adding a solidifying agent (agar 1. 5 3%). Prepared mainly in Petri dishes, but also in tubes and slopes. After growth the bacterial colonies are visible. e. g. blood agar, chocolate agar, Mac. Conkey agar. Semisolid agar: (soft agar): Contains small amounts of agar (0. 5 -0. 7%). Used to check for motility and also used as a transport media for fragile organisms. Can have semisolid agar in Petri dishes or in tubes. In tubes it is usually slanted to increase surface area.

LIQUID (BROTH): MOSTLY USED FOR BIOCHEMICAL TESTS (BLOOD CULTURE B, ROTH CULTURE). GROWTH OF BACTERIA IS SHOWN BY TURBIDITY IN MEDIUM. E. G. NUTRIENT BROTH, SELENITE F BROTH, ALKALINE PEPTONE WATER. PROPERTIES OF AGAR: SOME WHAT LIKE GELATIN. 0 C. IT MELTS AT 850 C AND SOLIDIFIES AT 40 -32 COMES AS SOLD POWDER AND THEN YOU ADD WATER TO IT.

TYPES OF CULTURE MEDIA Simple (basal, ordinary): Culture Media: are media that contain the basic nutrients (growth factors) that support the growth of bacteria without special nutrients, and they are used as basis of enriched media. E. g. Nutrient broth, nutrient agar, peptone water. They are for the growth of nonfastidious organisms like E. coli.

ENRICHED CULTURE MEDIA: ARE MEDIA THAT ARE ENRICHED WITH: WHOLE BLOOD E. G. BLOOD AGAR. LYSED BLOOD (HEATED TO 85 C) E. G. CHOCOLATE AGAR. SELECTIVE MEDIA: IT IS A MEDIA, WHICH CONTAINS SUBSTANCES THAT PREVENT OR SLOW THE GROWTH OF MICROORGANISMS OTHER THAN THE BACTERIA FOR WHICH THE MEDIA IS PREPARED FOR EXAMPLE EMB (EOSIN METHYLENE BLUE): ENTERIC ISOLATION MEDIA

DIFFERENTIAL MEDIA (INDICATORS): CONTAINS INDICATORS, DYES, ETC, TO DIFFERENTIATE MICROORGANISMS. E. G. MACCONKEY AGAR, WHICH CONTAINS NEUTRAL RED (PH INDICATOR) AND IS USED TO DIFFERENTIATE LACTOSE FERMENTER AND NON-LACTOSE FERMENTER. (E. G. E. COLIAND SALMONELLA).

COMMON MEDIA USED IN MICROBIOLOGY LABORATORY: Chocolate Agar: blood agar prepared by heating blood to 85 C until medium becomes brown or chocolate in color heating the blood releases broth X and V growth factors and also destroys the inhibitors of V factor. These factors are required for the growth of most species of Haemophilus and also Neisseria gonorrhoear. Mac. Conkey Agar: an inhibitory and differential medium used to distinguish lactose- fermenting Gram- negative organism from non fermentation. Crystal violet, bile salts and neutral red are inhibitor agent. neutral red is the PH indicator.

MANNITOL SALT AGAR ( MSA ): FOR SELECTIVE ISOLATION FOR COAGULASE POSITIVE, MANNITOL-FERMENTING STAPHYLOCOCCUS. MANNITOL FERMENTATION BY PATHOGENIC STAPHYLOCOCCI IS INDICATED BY A YELLOW HALO SURROUNDING THE COLONIES. SODIUM CHLORIDE IS THE INHIBITOR AGENTP. HENOL RED IS THE PH INDICATOR. MUELLER HINTON AGAR: RICH MEDIUM THAT SUPPORT THE GROWTH OF MOST MICROORGANISM. IT IS COMMONLY USED FOR ANTIBIOTIC SUSCEPTIBILITY TESTING: DISK DIFFUSION ANTIBIOTIC SUSCEPTIBILITY; ANTIBIOTIC SERUM LEVEL MEASUREMENTS; MBC DETERMINATION.

SALMONELLA SHIGELLA ( SS ) AGAR : ISOLATION AND DIFFERENTIAL MEDIUM FOR PATHOGENIC GRAM-NEGATIVE BACILLI IN PARTICULAR, SALMONELLA AND SHIGELLA. INHIBITOR FOR COLIFORMS. TRIPLE SUGAR IRON AGAR (TSI): THIS A KEY MEDIUM FOR USE IN BEGINNING THE IDENTIFICATION OF AGRAM- NEGATIVE BACILLI OF THE ENTERIC GROUP. IT CONTAINS GLUCOSE (0. 1% ), LACTOSE (1%), SUCROSE(1%). AND PEPTONE (2%) AS NUTRITIONAL SOURCES. SODIUM THIOSULFATE SERVES AS THE ELECTRON RECEPTOR FOR REDUCTION OF SULFUR AND PRODUCTION OF H 2 S. DETECTS FERMENTATION OF SUCROSE, LACTOSE, GLUCOSE, AS WELL AS PRODUCTION OF HYDROGEN SULFIDE AND /OR GAS . PHENOL RED IS THE PH INDICATOR; FERRIC AMMONIUM CITRATE IS H 2 S INDICATOR.

SINGLE MEDIA / MULTIPLE TESTS

SINGLE MEDIA / MULTIPLE TESTS Several media are designed to yield more than one biochemical reaction. Among the more commonly used media in this category are: SIM medium. Kliger's Iron agar (KIA). Triple Sugar Iron agar (TSIA). Lysine Iron agar (LIA). Motility Indole Ornithine (MIO) medium.

SIM MEDIUM INGREDIENTS q 0. 4% agar ( semisolid). q Peptone ( which rich in treptophan amino acid ). q Sodium thiosulfate q Ferrous ammonium sulfate. H 2 S INDICATOR The ingredients in SIM Medium enable the determination of three activities by which enteric bacteria can be differentiated. Sodium thiosulfate and ferrous ammonium sulfate for indication of hydrogen sulfide production. The ferrous ammonium sulfate reacts with H 2 S gas to produce ferrous sulfide, a black precipitate. The peptone is rich in tryptophan, which is attacked by certain microorganisms resulting in the production of indole. The indole is detected by the addition of Kovac’s reagent. Motility detection is possible due to the semisolid nature of the medium growth radiating out from the central stab line indicates that the test organism is motile.

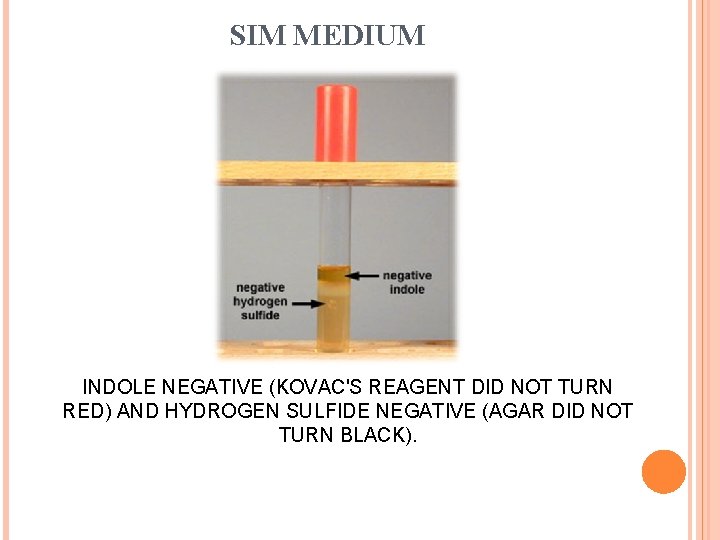
SIM MEDIUM INDOLE NEGATIVE (KOVAC'S REAGENT DID NOT TURN RED) AND HYDROGEN SULFIDE NEGATIVE (AGAR DID NOT TURN BLACK).

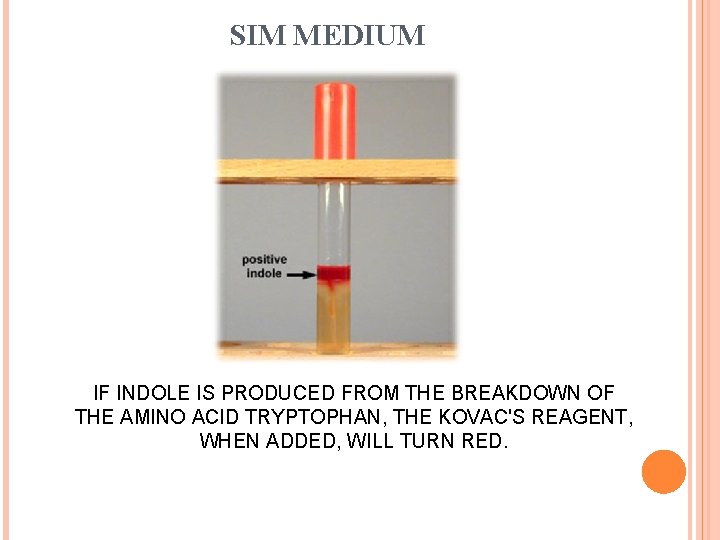
SIM MEDIUM IF INDOLE IS PRODUCED FROM THE BREAKDOWN OF THE AMINO ACID TRYPTOPHAN, THE KOVAC'S REAGENT, WHEN ADDED, WILL TURN RED.

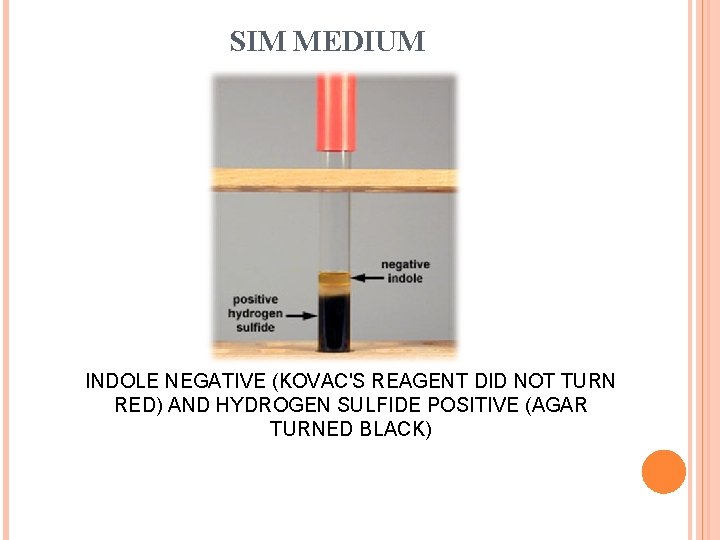
SIM MEDIUM INDOLE NEGATIVE (KOVAC'S REAGENT DID NOT TURN RED) AND HYDROGEN SULFIDE POSITIVE (AGAR TURNED BLACK)

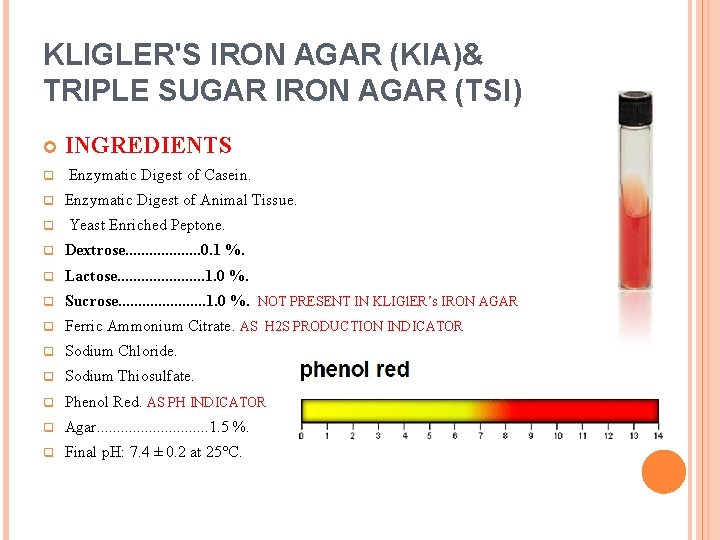
KLIGLER'S IRON AGAR (KIA)& TRIPLE SUGAR IRON AGAR (TSI) q q q INGREDIENTS Enzymatic Digest of Casein. Enzymatic Digest of Animal Tissue. Yeast Enriched Peptone. q Dextrose. . . . . 0. 1 %. q Lactose. . . . . 1. 0 %. q Sucrose. . . . . 1. 0 %. NOT PRESENT IN KLIGl. ER’s IRON AGAR q Ferric Ammonium Citrate. AS H 2 S PRODUCTION INDICATOR q Sodium Chloride. q Sodium Thiosulfate. q Phenol Red. AS PH INDICATOR q Agar. . . . 1. 5 %. q Final p. H: 7. 4 ± 0. 2 at 25°C.

PRINCIPLE Enzymatic Digest of Casein, Enzymatic Digest of Animal Tissue, and Yeast Enriched Peptone provide the nitrogen, carbon, and vitamins required for organism growth. Triple Sugar Iron Agar contains three carbohydrates, Dextrose, Lactose and Sucrose. When the carbohydrates are fermented, acid production is detected by the Phenol Red p. H indicator. Sodium Thiosulfate is reduced to hydrogen sulfide, and hydrogen sulfide reacts with an iron salt yielding the typical black iron sulfide. Ferric Ammonium Citrate is the hydrogen sulfide (H 2 S) indicator. Sodium Chloride maintains the osmotic balance of the medium. Agar is the solidifying agent.

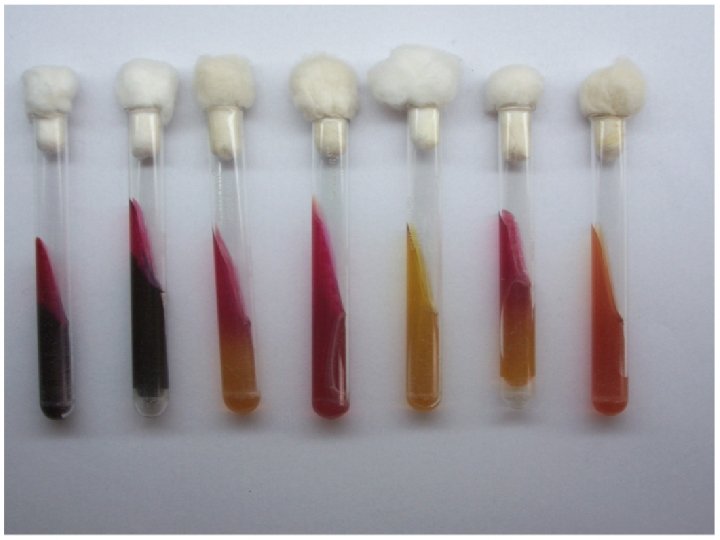
RESULTS An alkaline slant-acid butt (red/yellow) indicates fermentation of dextrose only. An acid slant-acid butt (yellow/yellow) indicates fermentation of dextrose, lactose and/or sucrose. An alkaline slant-alkaline butt (red/red) indicates dextrose , lactose or/& sucrose were not fermented (non-fermenter). Cracks, splits, or bubbles in medium indicate gas production. A black precipitate in butt indicates hydrogen sulfide production.

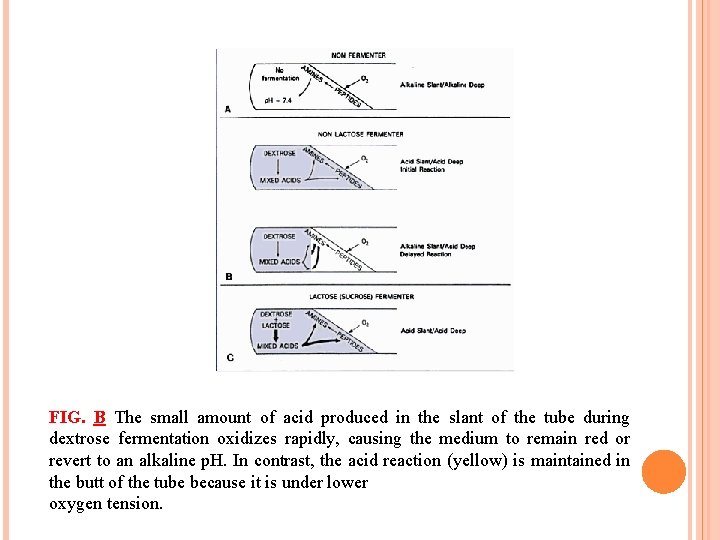
FIG. B The small amount of acid produced in the slant of the tube during dextrose fermentation oxidizes rapidly, causing the medium to remain red or revert to an alkaline p. H. In contrast, the acid reaction (yellow) is maintained in the butt of the tube because it is under lower oxygen tension.

Results (slant/butt) Symbol Interpretation Red/yellow K/A Glucose fermentation only; Peptone catabolized Yellow/yellow A/A Glucose and lactose and/or sucrose fermentation Red/red K/K No fermentation; Peptone catabolized Red/no color change K/NC No fermentation; Peptone used aerobically Yellow/yellow with bubbles A/A, G Glucose and lactose and/or sucrose fermentation; Gas produced Red/yellow with bubbles K/A, G Glucose fermentation only; Gas produced K/A, G, H 2 S Glucose fermentation only; Gas produced; H 2 S produced Red/yellow with black precipitate K/A, H 2 S Glucose fermentation only; H 2 S produced Yellow/yellow with black precipitate A/A, H 2 S Glucose and lactose and/or sucrose fermentation; H 2 S produced Red/yellow with bubbles and black precipitate


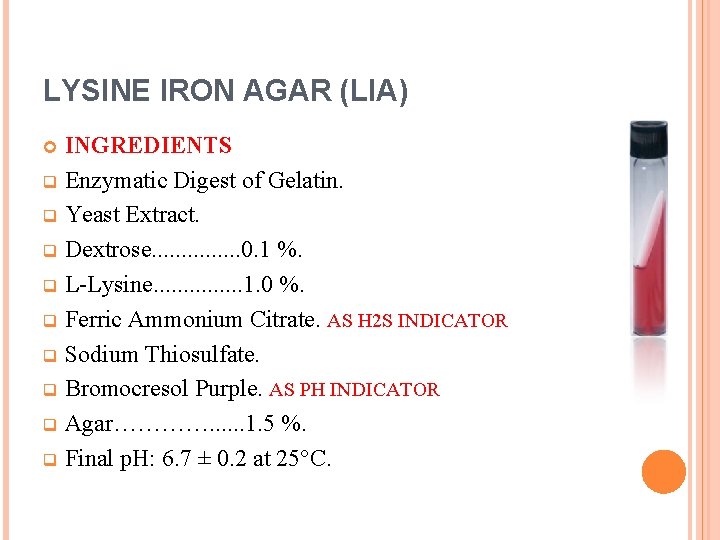
LYSINE IRON AGAR (LIA) INGREDIENTS q Enzymatic Digest of Gelatin. q Yeast Extract. q Dextrose. . . . 0. 1 %. q L-Lysine. . . . 1. 0 %. q Ferric Ammonium Citrate. AS H 2 S INDICATOR q Sodium Thiosulfate. q Bromocresol Purple. AS PH INDICATOR q Agar…………. . . 1. 5 %. q Final p. H: 6. 7 ± 0. 2 at 25°C.

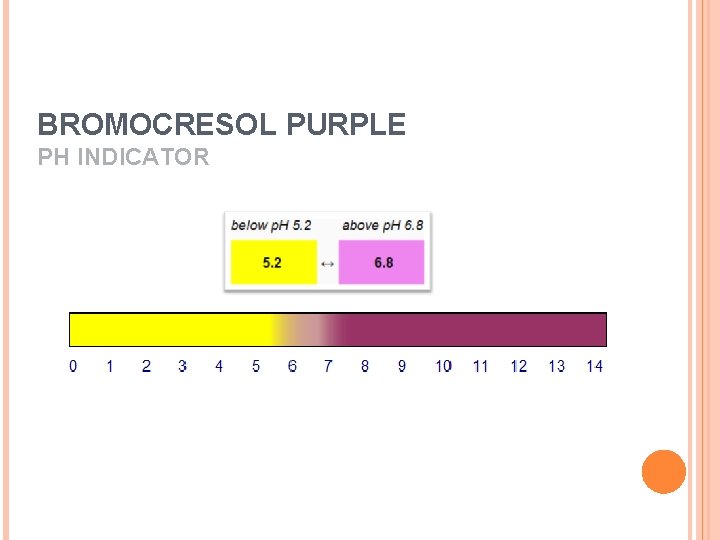
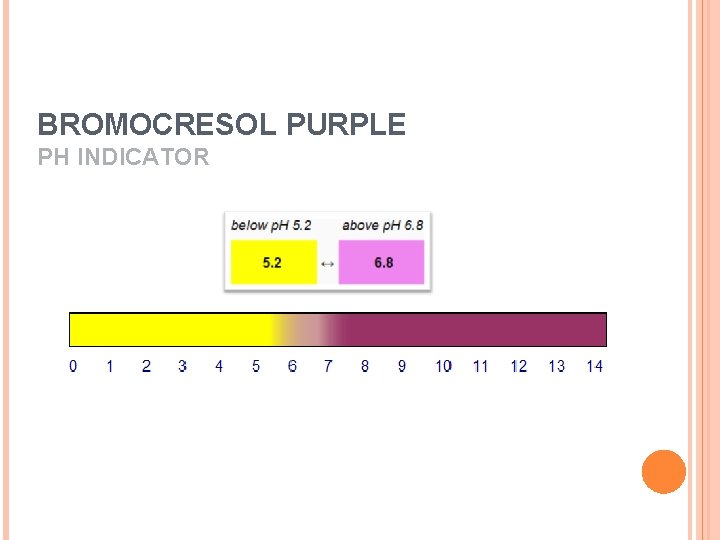
BROMOCRESOL PURPLE PH INDICATOR

LYSINE IRON AGAR (LIA)

MOTILITY INDOLE ORNITHINE (MIO) MEDIUM o INGREDIENTS Yeast Extract. q Peptone. q L-Ornithine……. . 0. 5%. q Dextrose. . . 0. 1%. q Agar ……………. 0. 4%. q Bromcresol Purple. PH INDICATOR q Final p. H 6. 5 ± 0. 2 at 25°C. q

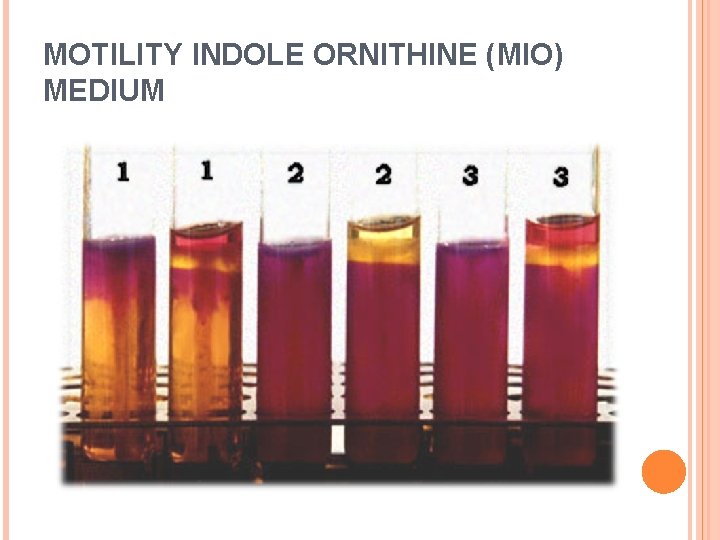
PRINCIPLE MIO Medium is prepared to provides three differentiating tests in one culture tube: motility, indole production, and ornithine decarboxylation. The principles of each are outlined below: o MOTILITY: Organisms are stabbed into the semisolid medium using a straight wire. If the organisms are motile, they will migrate from the stab line by means of their flagella, producing turbidity or cloudiness throughout the medium. Non-motile organisms grow only along the stab line, leaving the surrounding medium clear. o INDOLE: Tryptophan is an ingredient contained in the medium by the inclusion of peptones. If the organism possesses the enzyme tryptophanase, with the addition of Kovacs reagent, a red color will form at the top of the medium if indole is present. This reaction occurs as a result of the indole reacting with the aldehyde to yield a quinone or quinone-type structure, resulting in a red color in the alcohol layer. A negative test results in no color change.

PRINCIPLE CONTINUE…. o ORNITHINE: The medium also tests for the presence of the enzyme ornithine decarboxylase by including L-ornithine in the agar. If the organism possesses the enzyme, it will be activated in an acid environment created by the initial fermentation of glucose. Once the amino acid is decarboxylated, the by-product diamine putrescine is produced. The result is a shift in p. H to the alkaline range, turning the medium a dark purple. Organisms, which do not possess the enzyme, will stay in the acid range due to the fermentation, resulting in a yellow color in the medium.

BROMOCRESOL PURPLE PH INDICATOR

MOTILITY INDOLE ORNITHINE (MIO) MEDIUM