Live Attenuated Influenza Vaccine LAIV Administration in Schoolchildren





- Slides: 17

Live Attenuated Influenza Vaccine (LAIV) Administration in Schoolchildren Coincident with the 2003 -2004 Outbreak Provided Herd Immunity Against a Drifted Influenza Variant A/Fujian/411/2002 (H 3 N 2) Manjusha Gaglani, Pedro Piedra, Claudia Kozinetz, Gayla Herschler, Charles Fewlass, W. Paul Glezen Sponsors: NIAID, NIH and Med. Immune Inc. (provided Flu. Mist®)

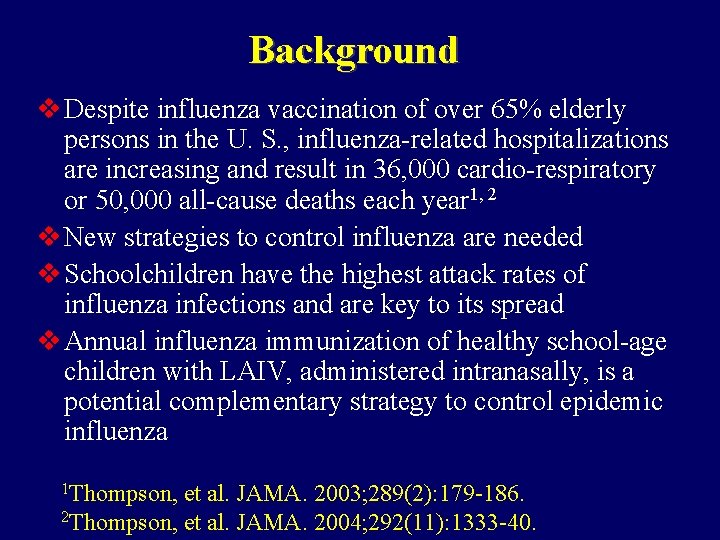
Background v Despite influenza vaccination of over 65% elderly persons in the U. S. , influenza-related hospitalizations are increasing and result in 36, 000 cardio-respiratory or 50, 000 all-cause deaths each year 1, 2 v New strategies to control influenza are needed v Schoolchildren have the highest attack rates of influenza infections and are key to its spread v Annual influenza immunization of healthy school-age children with LAIV, administered intranasally, is a potential complementary strategy to control epidemic influenza 1 Thompson, et al. JAMA. 2003; 289(2): 179 -186. 2 Thompson, et al. JAMA. 2004; 292(11): 1333 -40.

Control of Epidemic Influenza: Background v. Community-based immunization of 20 -25% of children, 18 months to 18 years of age, with the investigational LAIV, significantly reduced medically attended acute respiratory illnesses (MAARI) in adults over 35 years of age by 8 -18%3 during 3 consecutive epidemics from 1998 to 2001 3 Piedra, et al. Vaccine. 2005; 23(13): 1540 -8.

Control of Epidemic Influenza: Study Design v An open-label, non-randomized, community-based trial of annual influenza immunization of school-age children to effect herd immunity

Control of Epidemic Influenza: Objectives v. The primary objective is to determine the proportion of vaccinated school-age children needed to effect herd immunity (indirect effectiveness) against influenza v. The secondary objectives are to assess the direct effectiveness and safety of LAIV For Direct Effectiveness of LAIV in 2003 -2004, see Piedra, et al. Abstract # 726

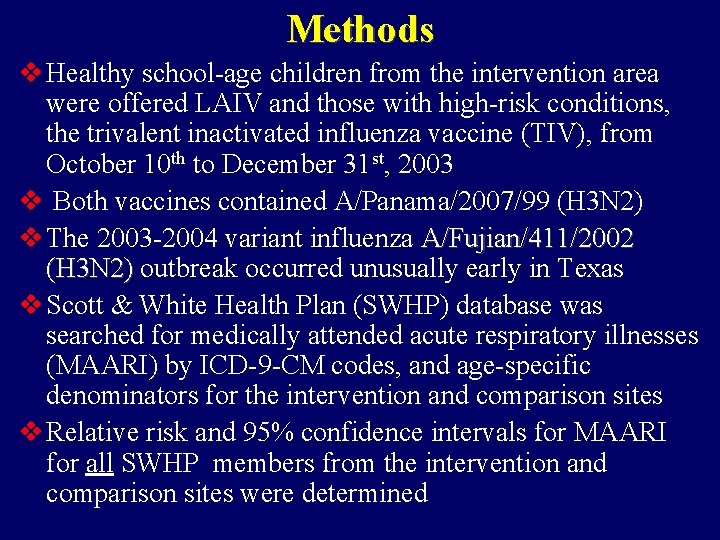
Methods v Healthy school-age children from the intervention area were offered LAIV and those with high-risk conditions, the trivalent inactivated influenza vaccine (TIV), from October 10 th to December 31 st, 2003 v Both vaccines contained A/Panama/2007/99 (H 3 N 2) v The 2003 -2004 variant influenza A/Fujian/411/2002 (H 3 N 2) outbreak occurred unusually early in Texas v Scott & White Health Plan (SWHP) database was searched for medically attended acute respiratory illnesses (MAARI) by ICD-9 -CM codes, and age-specific denominators for the intervention and comparison sites v Relative risk and 95% confidence intervals for MAARI for all SWHP members from the intervention and comparison sites were determined

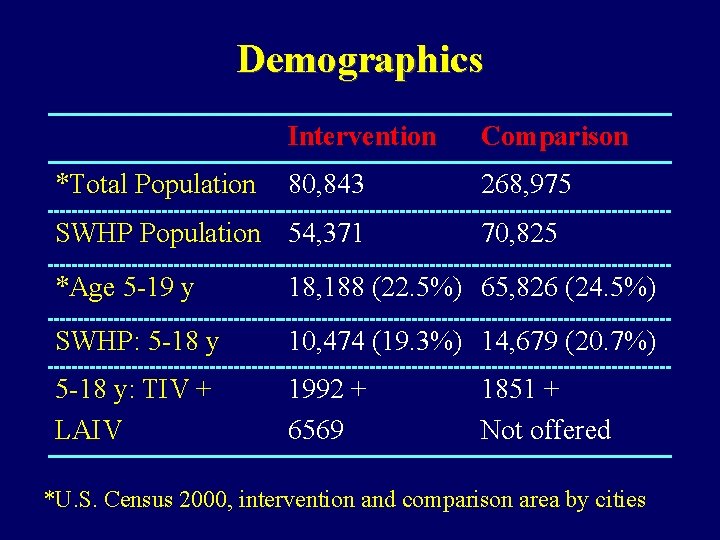
Demographics *Total Population Intervention Comparison 80, 843 268, 975 SWHP Population 54, 371 70, 825 *Age 5 -19 y 18, 188 (22. 5%) 65, 826 (24. 5%) SWHP: 5 -18 y 10, 474 (19. 3%) 14, 679 (20. 7%) 5 -18 y: TIV + LAIV 1992 + 6569 1851 + Not offered *U. S. Census 2000, intervention and comparison area by cities

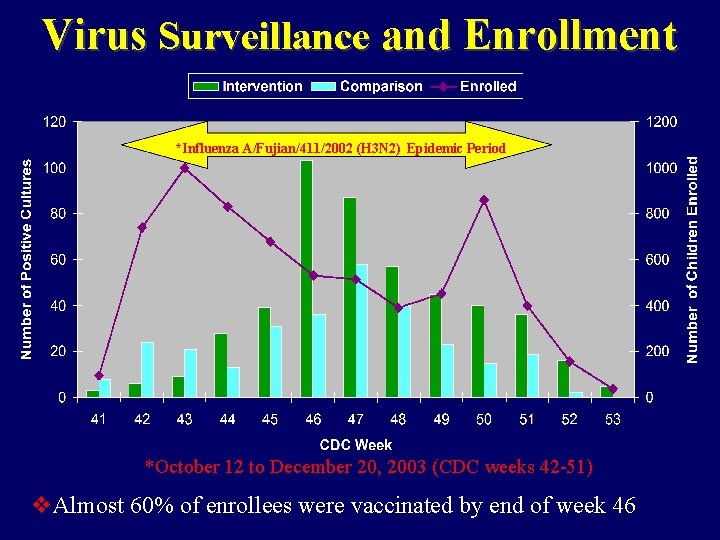
Virus Surveillance and Enrollment *Influenza A/Fujian/411/2002 (H 3 N 2) Epidemic Period *October 12 to December 20, 2003 (CDC weeks 42 -51) v. Almost 60% of enrollees were vaccinated by end of week 46

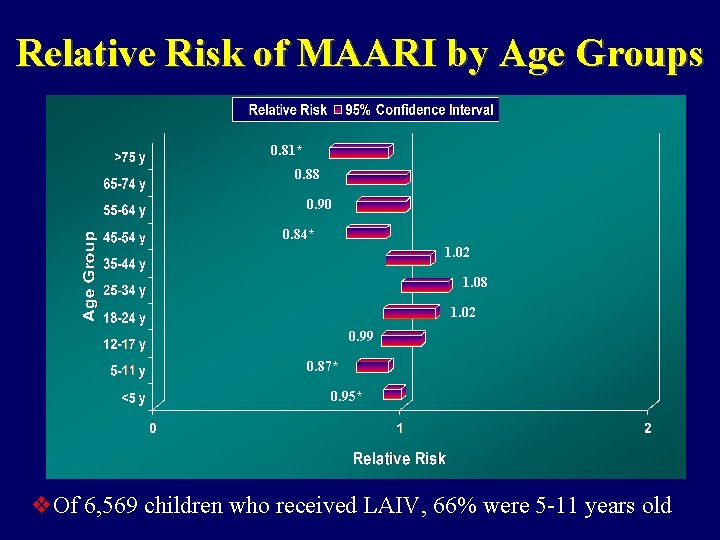
Relative Risk of MAARI by Age Groups 0. 81* 0. 88 0. 90 0. 84* 1. 02 1. 08 1. 02 0. 99 0. 87* 0. 95* v. Of 6, 569 children who received LAIV, 66% were 5 -11 years old

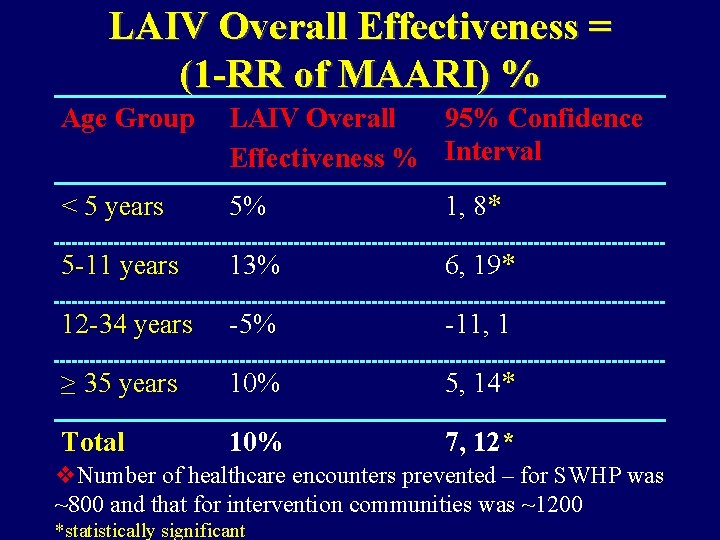
LAIV Overall Effectiveness = (1 -RR of MAARI) % Age Group LAIV Overall 95% Confidence Effectiveness % Interval < 5 years 5% 1, 8* 5 -11 years 13% 6, 19* 12 -34 years -5% -11, 1 ≥ 35 years 10% 5, 14* Total 10% 7, 12* v. Number of healthcare encounters prevented – for SWHP was ~800 and that for intervention communities was ~1200 *statistically significant

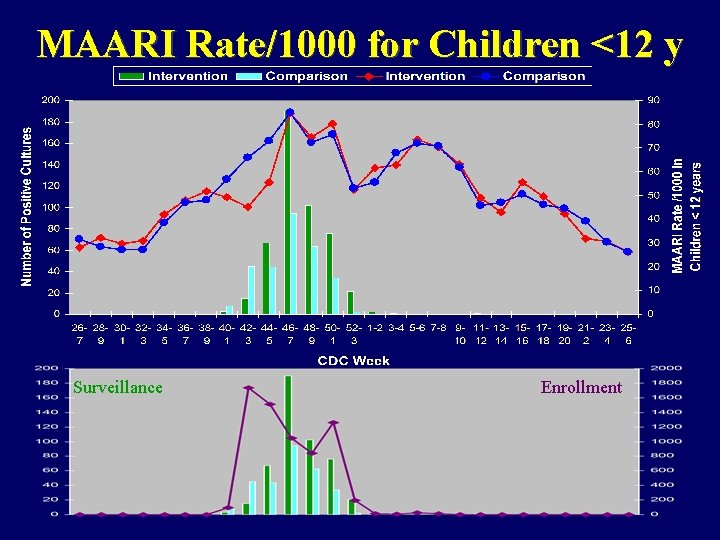
MAARI Rate/1000 for Children <12 y Surveillance Enrollment

Conclusion v. Community-based LAIV immunization of school-age children during an influenza outbreak provided herd immunity against a drifted variant influenza A by reducing its spread, possibly from early nonspecific and late specific immunity

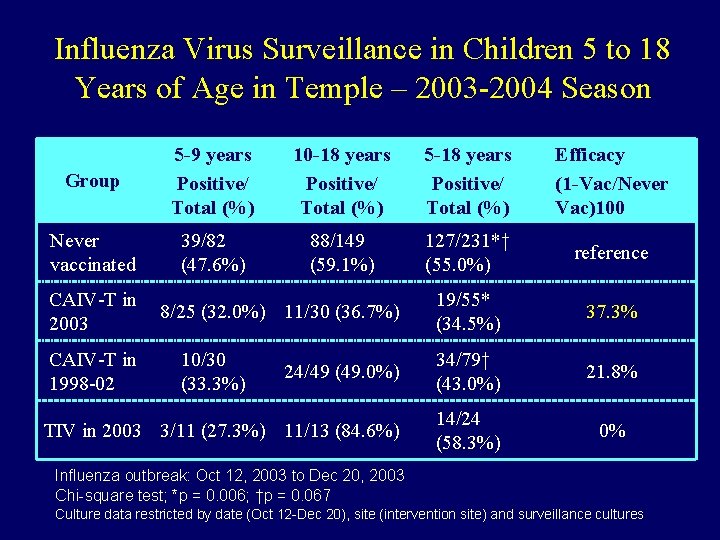
Influenza Virus Surveillance in Children 5 to 18 Years of Age in Temple – 2003 -2004 Season Group 5 -9 years Positive/ Total (%) 10 -18 years Positive/ Total (%) 5 -18 years Positive/ Total (%) Efficacy (1 -Vac/Never Vac)100 Never vaccinated 39/82 (47. 6%) 88/149 (59. 1%) 127/231*† (55. 0%) reference 19/55* (34. 5%) 37. 3% 24/49 (49. 0%) 34/79† (43. 0%) 21. 8% TIV in 2003 3/11 (27. 3%) 11/13 (84. 6%) 14/24 (58. 3%) 0% CAIV-T in 2003 CAIV-T in 1998 -02 8/25 (32. 0%) 11/30 (36. 7%) 10/30 (33. 3%) Influenza outbreak: Oct 12, 2003 to Dec 20, 2003 Chi-square test; *p = 0. 006; †p = 0. 067 Culture data restricted by date (Oct 12 -Dec 20), site (intervention site) and surveillance cultures

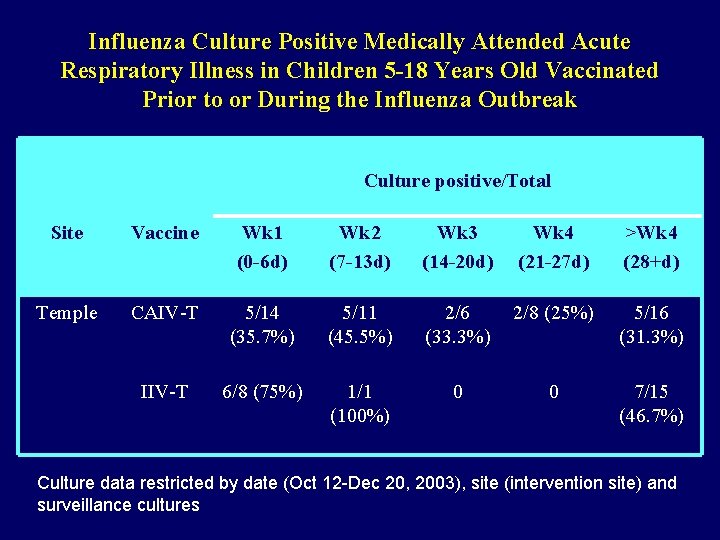
Influenza Culture Positive Medically Attended Acute Respiratory Illness in Children 5 -18 Years Old Vaccinated Prior to or During the Influenza Outbreak Culture positive/Total Site Vaccine Wk 1 (0 -6 d) Wk 2 (7 -13 d) Wk 3 (14 -20 d) Wk 4 (21 -27 d) >Wk 4 (28+d) Temple CAIV-T 5/14 (35. 7%) 5/11 (45. 5%) 2/6 (33. 3%) 2/8 (25%) 5/16 (31. 3%) IIV-T 6/8 (75%) 1/1 (100%) 0 0 7/15 (46. 7%) Culture data restricted by date (Oct 12 -Dec 20, 2003), site (intervention site) and surveillance cultures

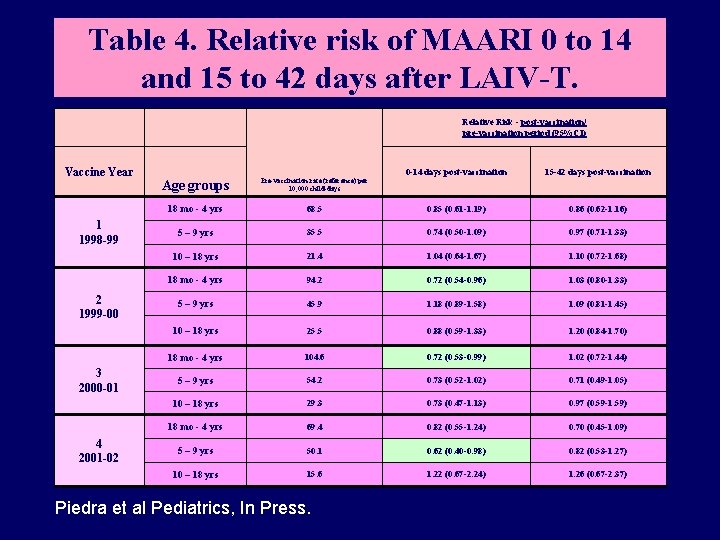
Table 4. Relative risk of MAARI 0 to 14 and 15 to 42 days after LAIV-T. Relative Risk - post-vaccination/ pre-vaccination period (95%CI) Vaccine Year 1 1998 -99 2 1999 -00 3 2000 -01 4 2001 -02 0 -14 days post-vaccination 15 -42 days post-vaccination Age groups Pre-vaccination rate (reference) per 10, 000 child-days 18 mo - 4 yrs 68. 5 0. 85 (0. 61 -1. 19) 0. 86 (0. 62 -1. 16) 5 – 9 yrs 35. 5 0. 74 (0. 50 -1. 09) 0. 97 (0. 71 -1. 33) 10 – 18 yrs 21. 4 1. 04 (0. 64 -1. 67) 1. 10 (0. 72 -1. 68) 18 mo - 4 yrs 94. 2 0. 72 (0. 54 -0. 96) 1. 03 (0. 80 -1. 33) 5 – 9 yrs 45. 9 1. 18 (0. 89 -1. 58) 1. 09 (0. 81 -1. 45) 10 – 18 yrs 25. 5 0. 88 (0. 59 -1. 33) 1. 20 (0. 84 -1. 70) 18 mo - 4 yrs 104. 6 0. 72 (0. 53 -0. 99) 1. 02 (0. 72 -1. 44) 5 – 9 yrs 54. 2 0. 73 (0. 52 -1. 02) 0. 71 (0. 49 -1. 05) 10 – 18 yrs 29. 3 0. 73 (0. 47 -1. 13) 0. 97 (0. 59 -1. 59) 18 mo - 4 yrs 69. 4 0. 82 (0. 55 -1. 24) 0. 70 (0. 45 -1. 09) 5 – 9 yrs 50. 1 0. 62 (0. 40 -0. 98) 0. 82 (0. 53 -1. 27) 10 – 18 yrs 15. 6 1. 22 (0. 67 -2. 24) 1. 26 (0. 67 -2. 37) Piedra et al Pediatrics, In Press.

Summary § In 2003 – 2004, the delivery of influenza vaccine was coincident with occurrence of the epidemic caused by A/ Fujian (H 3 N 2), a new variant significantly different from the vaccine virus. §Virtually immediate protection provided by LAIV-T resulted in 10% reduction in MAARI at the intervention site; no protection could be attributed to inactivated vaccine. §The reduction of MAARI at the intervention site was most pronounced in the early stage of the epidemic when large numbers of children received LAIV. §This and previous safety data showing decreased MAARI 0 -14 days after LAIV supports the hypothesis of non-specific (innate) protection against viral infection.

Acknowledgement* Baylor CM Don Wilson Bob Fader Emory U Pedro Piedra Robin Cameron Felicia Bagwell Ira Longini W Paul Glezen Carolyn Connally Mark Riggs Betz Halloran Claudia Kozinetz Art Mow N Zimmerman U of Texas Kirti Patel Matthew Watts Pat Kirkpatrick Scott Lilibridge Terri Bosshard Steven Slater Scott Clark Med. Immune Sneha Thaker Chris Morgan Wendy White Jeffrey Stoddard Sheldon Kaplan Jeff Wilson Rinska Flores Caroleen Becker Scott & White Alice Linder Sonia Holleman NIH Gayla Herschler Olivia Ametane TX Dept Health Linda Lambert Charles Fewlass Pat Smith. Peggy Wright Sonnie Kim Hope Gonzales Debbie Miller Neil Pascoe Tem-Bel Health &Wellness Coalition *Communities of Temple-Belton and surrounding area
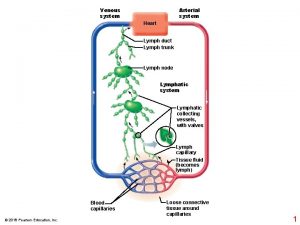
 Viral gastroenteritis
Viral gastroenteritis Influenza vaccine dosage chart 2019-2020
Influenza vaccine dosage chart 2019-2020 Flaplike minivalve
Flaplike minivalve Vaccine administration technique
Vaccine administration technique 7 rights of vaccine administration
7 rights of vaccine administration Vaccine administration technique
Vaccine administration technique Influenza
Influenza Influenza virus replication
Influenza virus replication Influenza ww1
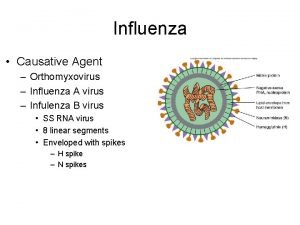
Influenza ww1 Influenza causative agent
Influenza causative agent Stomach flu vs influenza
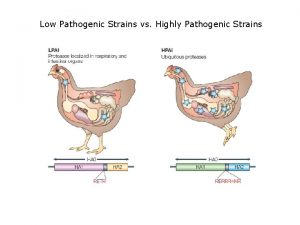
Stomach flu vs influenza Low pathogenic avian influenza
Low pathogenic avian influenza The great influenza rhetorical analysis
The great influenza rhetorical analysis Is influenza a airborne disease
Is influenza a airborne disease Olfactory mucosa
Olfactory mucosa Fibertel
Fibertel Spinte metallostatiche
Spinte metallostatiche Https://quizlet.com live
Https://quizlet.com live