Literature Review Chitin and Chitosan From Nature to



![What’s next: Biotechnology Enzyme immobilization [1] l Specific, efficient, operate at mild conditions l What’s next: Biotechnology Enzyme immobilization [1] l Specific, efficient, operate at mild conditions l](https://slidetodoc.com/presentation_image/5d40e6258d0ee246d3d070f79f4b2ba9/image-16.jpg)
![Chitin in Fungi Cell Walls What are fungi? [1] Fungi have the following characteristics: Chitin in Fungi Cell Walls What are fungi? [1] Fungi have the following characteristics:](https://slidetodoc.com/presentation_image/5d40e6258d0ee246d3d070f79f4b2ba9/image-19.jpg)
![Chitin in Arthropods Cuticles What are arthropods? [1] The arthropods constitute over 90% of Chitin in Arthropods Cuticles What are arthropods? [1] The arthropods constitute over 90% of](https://slidetodoc.com/presentation_image/5d40e6258d0ee246d3d070f79f4b2ba9/image-21.jpg)

- Slides: 37

Literature Review: Chitin and Chitosan From Nature to Technology Po-Yu Chen Materials Science & Engineering University of California, San Diego May 3 rd , 2006

Outline 1. Introduction 2. Production of Chitin/Chitosan 3. Selected Applications 4. Chitin in Nature 5. Conclusions

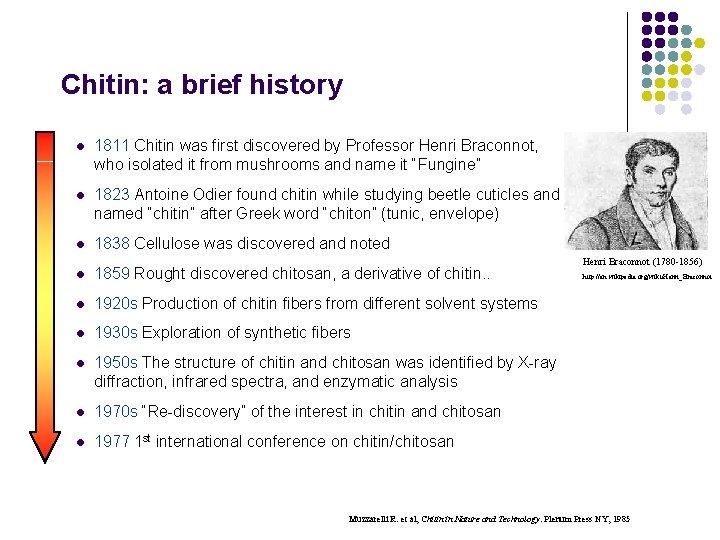
Chitin: a brief history l 1811 Chitin was first discovered by Professor Henri Braconnot, who isolated it from mushrooms and name it “Fungine” l 1823 Antoine Odier found chitin while studying beetle cuticles and named “chitin” after Greek word “chiton” (tunic, envelope) l 1838 Cellulose was discovered and noted l 1859 Rought discovered chitosan, a derivative of chitin. . l 1920 s Production of chitin fibers from different solvent systems l 1930 s Exploration of synthetic fibers l 1950 s The structure of chitin and chitosan was identified by X-ray diffraction, infrared spectra, and enzymatic analysis l 1970 s “Re-discovery” of the interest in chitin and chitosan l 1977 1 st international conference on chitin/chitosan Henri Braconnot (1780 -1856) http: //en. wikipedia. org/wiki/Henri_Braconnot Muzzarelli R. et al, Chitin in Nature and Technology. Plenum Press NY, 1985

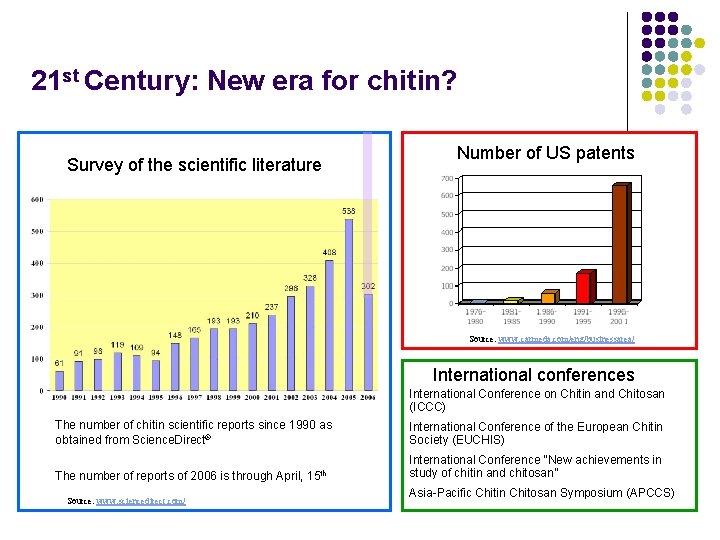
21 st Century: New era for chitin? Survey of the scientific literature Number of US patents Source: www. carmeda. com/eng/businessarea/ International conferences International Conference on Chitin and Chitosan (ICCC) The number of chitin scientific reports since 1990 as obtained from Science. Direct® International Conference of the European Chitin Society (EUCHIS) The number of reports of 2006 is through April, 15 th International Conference “New achievements in study of chitin and chitosan” Source: www. sciencedirect. com/ Asia-Pacific Chitin Chitosan Symposium (APCCS)

Chitin: a promising material Unique characteristics of chitin and chitosan: v Biocompatible v Biodegradable v Non-toxic v Remarkable affinity to proteins v Ability to be functionalized v Renewable v Abundant Muzzarelli R. et al, Chitin in Nature and Technology. Plenum Press NY, 1985

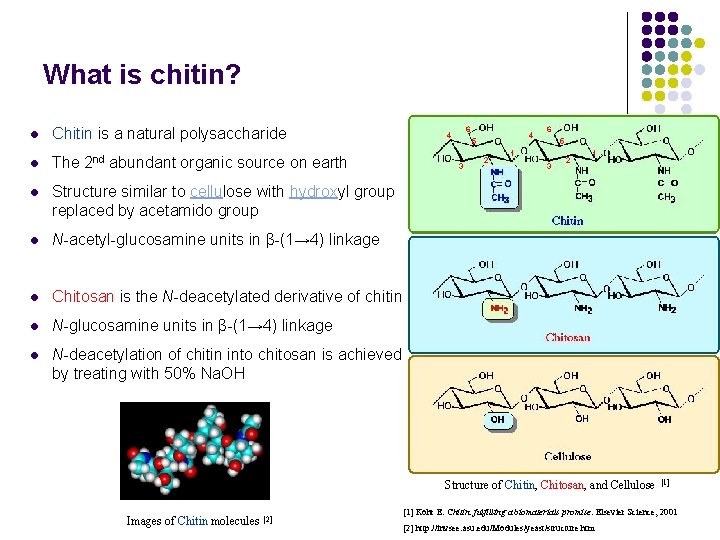
What is chitin? l Chitin is a natural polysaccharide l The 2 nd abundant organic source on earth l Structure similar to cellulose with hydroxyl group replaced by acetamido group l N-acetyl-glucosamine units in β-(1→ 4) linkage l Chitosan is the N-deacetylated derivative of chitin l N-glucosamine units in β-(1→ 4) linkage l N-deacetylation of chitin into chitosan is achieved by treating with 50% Na. OH 6 4 4 5 3 2 6 5 1 3 2 1 Structure of Chitin, Chitosan, and Cellulose Images of Chitin molecules [2] [1] Kohr E. Chitin: fulfilling a biomaterials promise. Elsevier Science, 2001 [2] http: //invsee. asu. edu/Modules/yeast/structure. htm

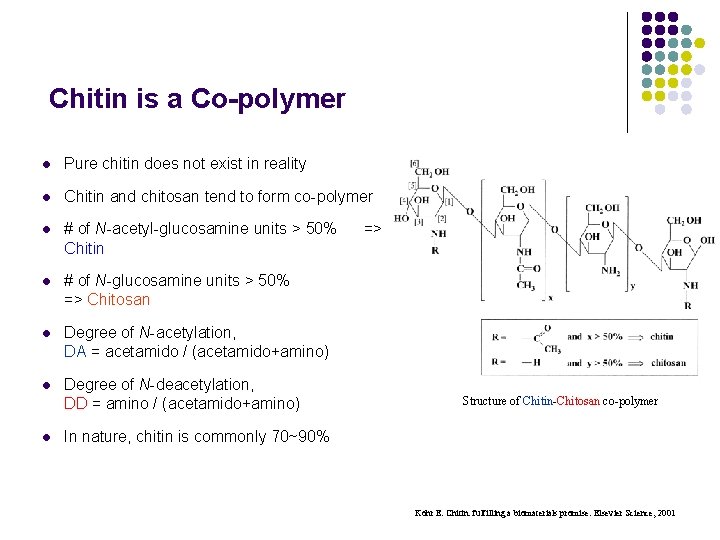
Chitin is a Co-polymer l Pure chitin does not exist in reality l Chitin and chitosan tend to form co-polymer l # of N-acetyl-glucosamine units > 50% => Chitin l # of N-glucosamine units > 50% => Chitosan l Degree of N-acetylation, DA = acetamido / (acetamido+amino) l Degree of N-deacetylation, DD = amino / (acetamido+amino) l Structure of Chitin-Chitosan co-polymer In nature, chitin is commonly 70~90% Kohr E. Chitin: fulfilling a biomaterials promise. Elsevier Science, 2001

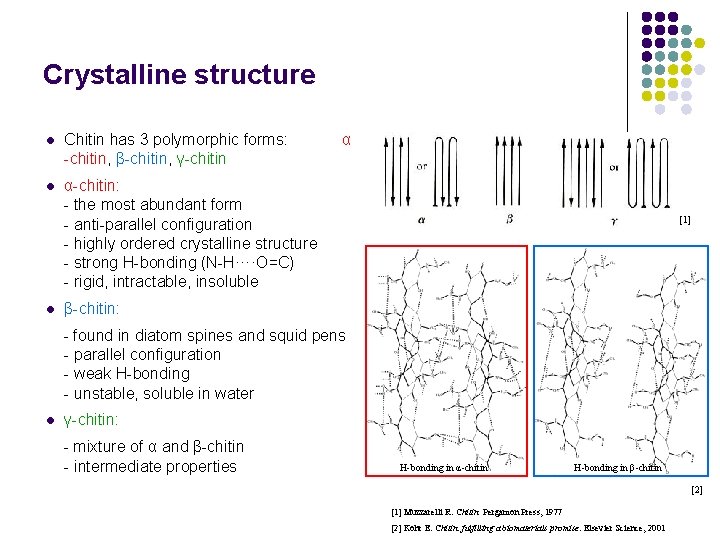
Crystalline structure l Chitin has 3 polymorphic forms: α -chitin, β-chitin, γ-chitin l α-chitin: - the most abundant form - anti-parallel configuration - highly ordered crystalline structure - strong H-bonding (N-H····O=C) - rigid, intractable, insoluble l [1] β-chitin: - found in diatom spines and squid pens - parallel configuration - weak H-bonding - unstable, soluble in water l γ-chitin: - mixture of α and β-chitin - intermediate properties H-bonding in α-chitin H-bonding in β-chitin [2] [1] Muzzarelli R. Chitin. Pergamon Press, 1977 [2] Kohr E. Chitin: fulfilling a biomaterials promise. Elsevier Science, 2001

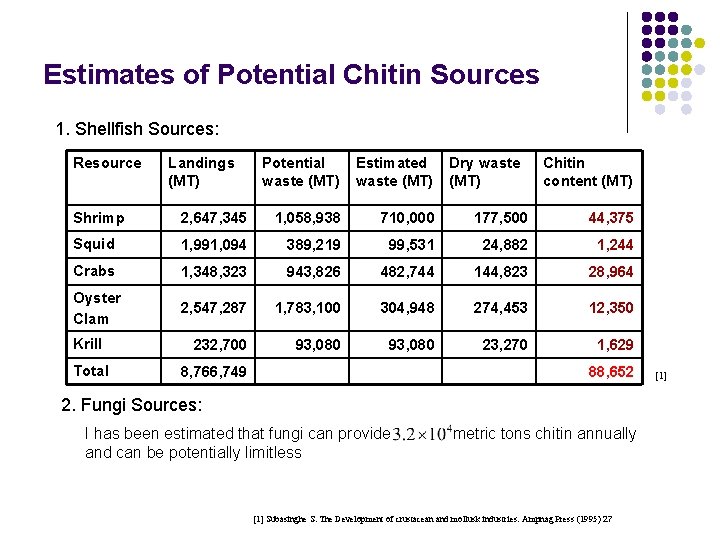
Estimates of Potential Chitin Sources 1. Shellfish Sources: Resource Landings (MT) Potential waste (MT) Estimated waste (MT) Dry waste (MT) Chitin content (MT) Shrimp 2, 647, 345 1, 058, 938 710, 000 177, 500 44, 375 Squid 1, 991, 094 389, 219 99, 531 24, 882 1, 244 Crabs 1, 348, 323 943, 826 482, 744 144, 823 28, 964 Oyster Clam 2, 547, 287 1, 783, 100 304, 948 274, 453 12, 350 232, 700 93, 080 23, 270 1, 629 Krill Total 8, 766, 749 88, 652 2. Fungi Sources: I has been estimated that fungi can provide metric tons chitin annually and can be potentially limitless [1] Subasinghe S. The Development of crustacean and mollusk industries. Ampnag Press (1995) 27 [1]

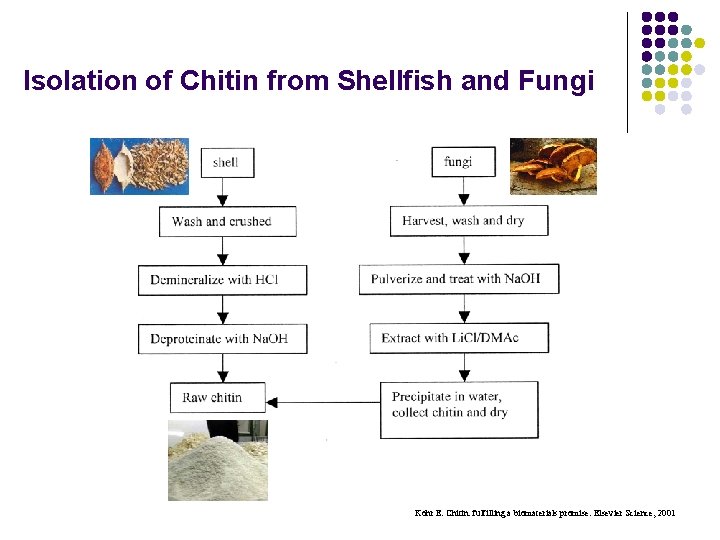
Isolation of Chitin from Shellfish and Fungi Kohr E. Chitin: fulfilling a biomaterials promise. Elsevier Science, 2001

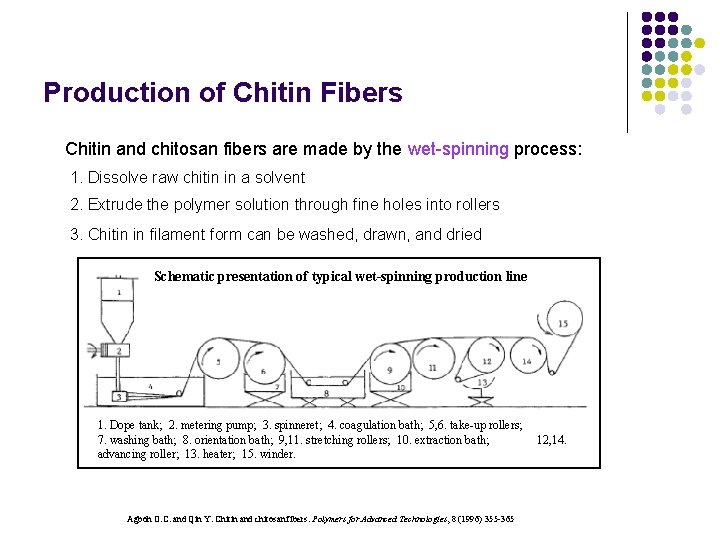
Production of Chitin Fibers Chitin and chitosan fibers are made by the wet-spinning process: 1. Dissolve raw chitin in a solvent 2. Extrude the polymer solution through fine holes into rollers 3. Chitin in filament form can be washed, drawn, and dried Schematic presentation of typical wet-spinning production line 1. Dope tank; 2. metering pump; 3. spinneret; 4. coagulation bath; 5, 6. take-up rollers; 7. washing bath; 8. orientation bath; 9, 11. stretching rollers; 10. extraction bath; advancing roller; 13. heater; 15. winder. Agboh O. C. and Qin Y. Chitin and chitosan fibers. Polymers for Advanced Technologies, 8 (1996) 355 -365 12, 14.

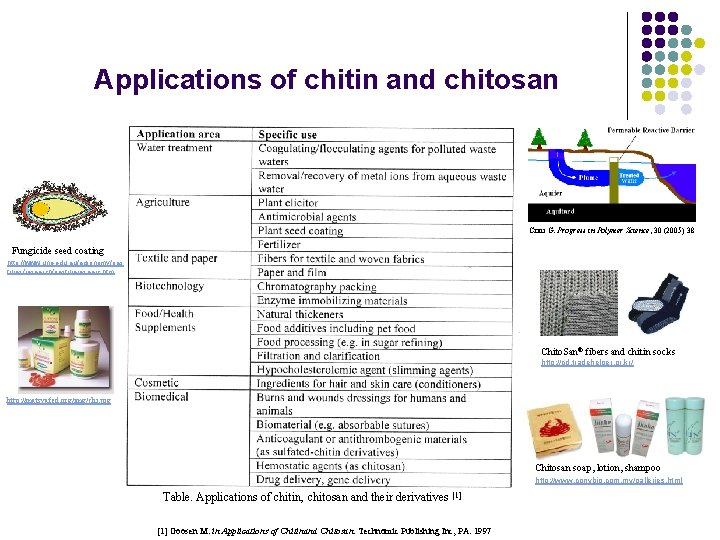
Applications of chitin and chitosan Crini G. Progress in Polymer Science, 30 (2005) 38 Fungicide seed coating http: //www. une. edu. au/agronomy/pas tures/research/pastureresearc. htm Chito. San® fibers and chitin socks http: //cd. tradehelper. or. kr/ http: //matsyafed. org/img/chi. jpg Chitosan soap, lotion, shampoo http: //www. conybio. com. my/galleries. html Table. Applications of chitin, chitosan and their derivatives [1] Goosen M. in Applications of Chitinand Chitosan. Technomic Publishing Inc, PA. 1997

Biomedical Applications Wound Dressing Wound dressings are used to protect wound skin form insult, contamination and infection l Chitin-based wound dressings l - Increase dermal regeneration - Accelerate wound healing - Prevent bacteria infiltration - Avoid water loss l [1] Chitosan wound dressings Chitin surgical threads - strong, flexible, decompose after the heals Anticoagulation l Anticoagulation is essential for open-heart surgery and kidney dialysis l Preventing blood from clotting during the surgery l Sulfated chitin derivatives have good anticoagulant activity [2] Cell culture compatibility ranking of wound dressing materials [1] Kohr E. Chitin: fulfilling a biomaterials promise. Elsevier Science, 2001 [2] Khor E. Lee Y. L. Implantable applications of chitin and chtosan. Biomaterials 24 (2003) 2339

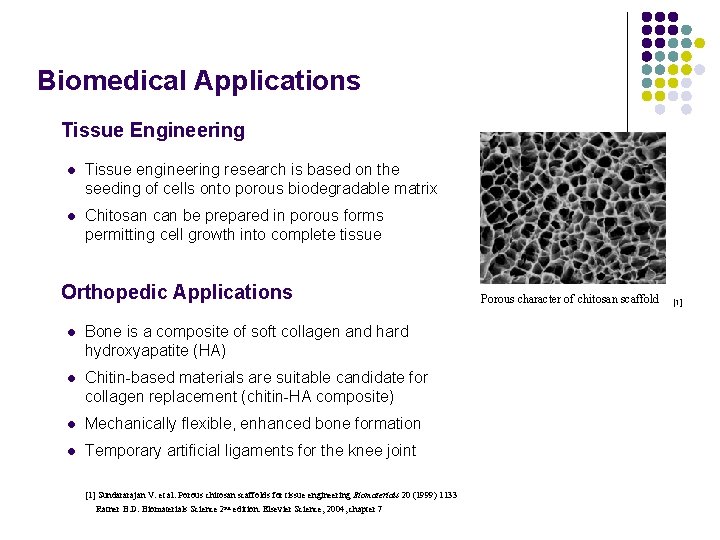
Biomedical Applications Tissue Engineering l Tissue engineering research is based on the seeding of cells onto porous biodegradable matrix l Chitosan can be prepared in porous forms permitting cell growth into complete tissue Orthopedic Applications l Bone is a composite of soft collagen and hard hydroxyapatite (HA) l Chitin-based materials are suitable candidate for collagen replacement (chitin-HA composite) l Mechanically flexible, enhanced bone formation l Temporary artificial ligaments for the knee joint [1] Sundararajan V. et al. Porous chitosan scaffolds for tissue engineering Biomaterials 20 (1999) 1133 Ratner B. D. Biomaterials Science 2 nd edition. Elsevier Science, 2004, chapter 7 Porous character of chitosan scaffold 50μm [1]

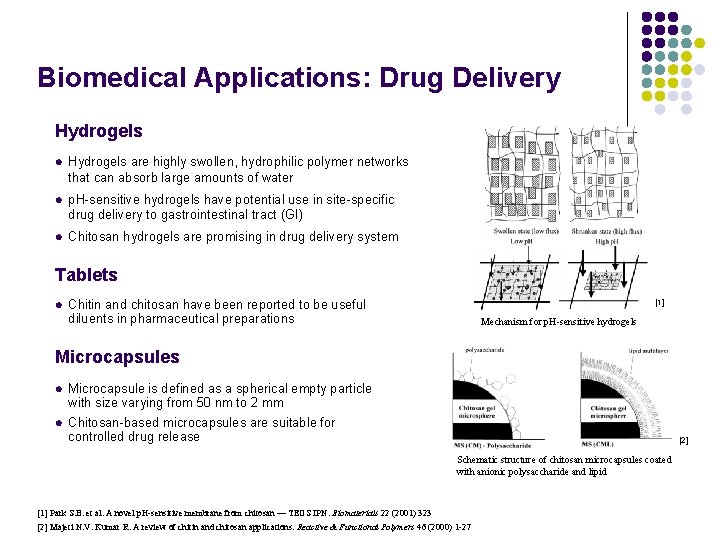
Biomedical Applications: Drug Delivery Hydrogels l Hydrogels are highly swollen, hydrophilic polymer networks that can absorb large amounts of water l p. H-sensitive hydrogels have potential use in site-specific drug delivery to gastrointestinal tract (GI) l Chitosan hydrogels are promising in drug delivery system Tablets l Chitin and chitosan have been reported to be useful diluents in pharmaceutical preparations [1] Mechanism for p. H-sensitive hydrogels Microcapsules l Microcapsule is defined as a spherical empty particle with size varying from 50 nm to 2 mm l Chitosan-based microcapsules are suitable for controlled drug release [2] Schematic structure of chitosan microcapsules coated with anionic polysaccharide and lipid [1] Park S. B. et al. A novel p. H-sensitive membrane from chitosan — TEOS IPN. Biomaterials 22 (2001) 323 [2] Majeti N. V. Kumar R. A review of chitin and chitosan applications. Reactive & Functional Polymers 46 (2000) 1 -27
![Whats next Biotechnology Enzyme immobilization 1 l Specific efficient operate at mild conditions l What’s next: Biotechnology Enzyme immobilization [1] l Specific, efficient, operate at mild conditions l](https://slidetodoc.com/presentation_image/5d40e6258d0ee246d3d070f79f4b2ba9/image-16.jpg)
What’s next: Biotechnology Enzyme immobilization [1] l Specific, efficient, operate at mild conditions l Unstable, sensitive after isolation and purification l Chitin and chitosan-based materials are suitable enzyme immobilizers Purves W. K. et al Life: The Science of Biology 6 th edition. Sinauer Associates Inc. (2001) - Biocompatible - Biodegradable - High affinity to protein - Reactive functional group Gene Delivery [2] l Viral gene delivery / Non-Viral gene delivery l Viral: high transfection efficiency, dangerous l Non-Viral: low transfection efficiency, safer l Chitosan-DNA complexes can be optimized to enhance the transfection efficiency http: //ghr. nlm. nih. gov/dynamic. Images/understand. Genetics/gene_therapy. jpg [1] Krajewska B. Application of chitin and chitosan-based materials for enzyme immobilizations Enzyme and Microbial Technology 35 (2004) 126 [2] Shi C. et al Therapeutic potential of chitosan and Its derivatives in regenerative medicine. Journal of Surgical Research (2006) In press

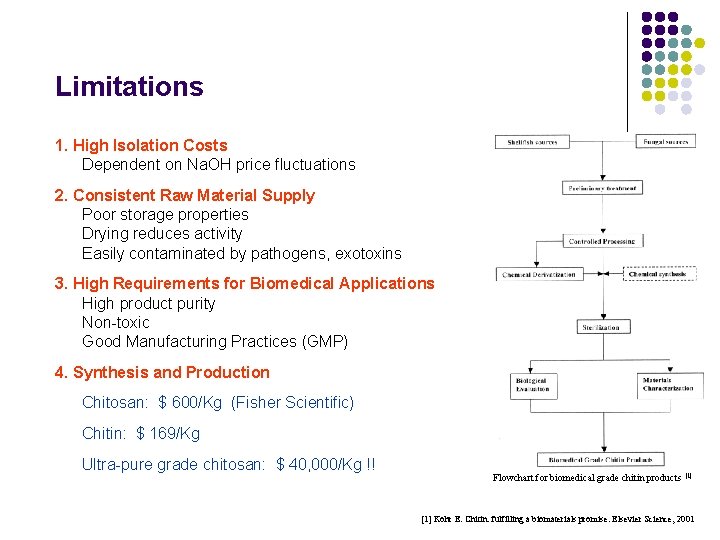
Limitations 1. High Isolation Costs Dependent on Na. OH price fluctuations 2. Consistent Raw Material Supply Poor storage properties Drying reduces activity Easily contaminated by pathogens, exotoxins 3. High Requirements for Biomedical Applications High product purity Non-toxic Good Manufacturing Practices (GMP) 4. Synthesis and Production Chitosan: $ 600/Kg (Fisher Scientific) Chitin: $ 169/Kg Ultra-pure grade chitosan: $ 40, 000/Kg !! Flowchart for biomedical grade chitin products [1] Kohr E. Chitin: fulfilling a biomaterials promise. Elsevier Science, 2001

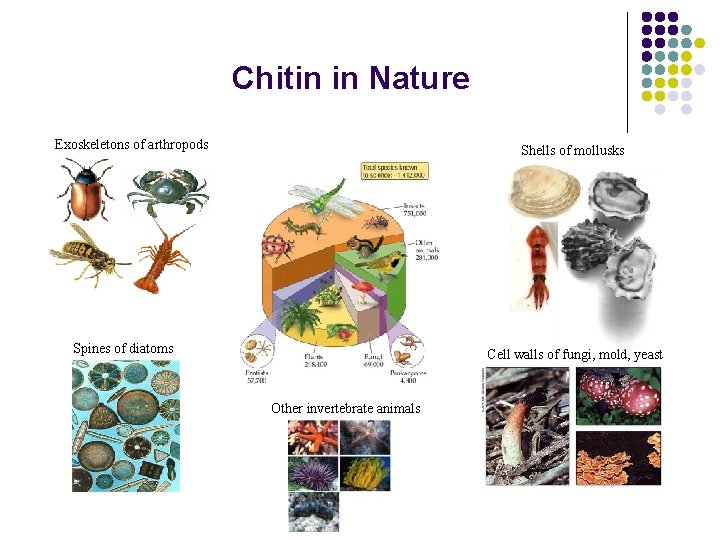
Chitin in Nature Exoskeletons of arthropods Shells of mollusks Spines of diatoms Cell walls of fungi, mold, yeast Other invertebrate animals
![Chitin in Fungi Cell Walls What are fungi 1 Fungi have the following characteristics Chitin in Fungi Cell Walls What are fungi? [1] Fungi have the following characteristics:](https://slidetodoc.com/presentation_image/5d40e6258d0ee246d3d070f79f4b2ba9/image-19.jpg)
Chitin in Fungi Cell Walls What are fungi? [1] Fungi have the following characteristics: l Their main body is in the form of thin strands called mycelium l Can not produce their own food through photosynthesis l The major decomposer of organic matter l Their cell walls are made mostly of chitin http: //www. anselm. edu/homepage/jpitocch/genbios/31 -01 -Fungal. Mycelia-L. jpg Chitin in fungi l Fungal chitin occurs as randomly oriented microfibrils typically 10 -25 nm in diameter and 2~3 μm long l Chitin is covalently linked to other polysaccharides, such as glucans, and forms chitin-glucan complex l The chitin content in fungi varies from 0. 5% in yeast to 50% on filamentous fungi species [1] Purves W. K. et al Life: The Science of Biology 6 th edition. Sinauer Associates Inc. (2001) [2] Herth W. Zugenmaier P. Microbiology Letters, (1986) 263 Muzzarelli R. et al, Chitin in Nature and Technology. Plenum Press NY, 1985 0. 1 μm SEM micrograph of chitin microfibrils (Poterioochromonas stipitata) [2]

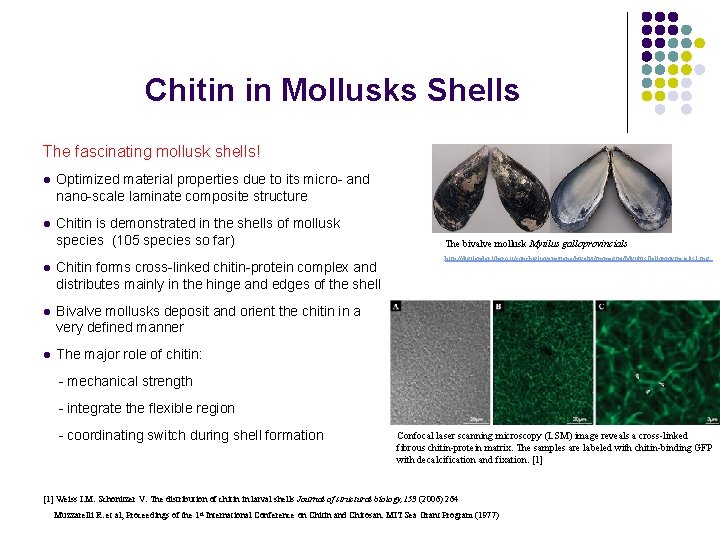
Chitin in Mollusks Shells The fascinating mollusk shells! l Optimized material properties due to its micro- and nano-scale laminate composite structure l Chitin is demonstrated in the shells of mollusk species (105 species so far) l Chitin forms cross-linked chitin-protein complex and distributes mainly in the hinge and edges of the shell l Bivalve mollusks deposit and orient the chitin in a very defined manner l The major role of chitin: The bivalve mollusk Mytilus galloprovincials http: //digilander. libero. it/conchiglieveneziane/bivalvi/immagini/Mytilus. Galloprovincialis 1. jpg - mechanical strength - integrate the flexible region - coordinating switch during shell formation Confocal laser scanning microscopy (LSM) image reveals a cross-linked fibrous chitin-protein matrix. The samples are labeled with chitin-binding GFP with decalcification and fixation. [1] Weiss I. M. Schonitzer V. The distribution of chitin in larval shells Journal of structural biology, 153 (2006) 264 Muzzarelli R. et al, Proceedings of the 1 st International Conference on Chitin and Chitosan. MIT Sea Grant Program (1977)
![Chitin in Arthropods Cuticles What are arthropods 1 The arthropods constitute over 90 of Chitin in Arthropods Cuticles What are arthropods? [1] The arthropods constitute over 90% of](https://slidetodoc.com/presentation_image/5d40e6258d0ee246d3d070f79f4b2ba9/image-21.jpg)
Chitin in Arthropods Cuticles What are arthropods? [1] The arthropods constitute over 90% of the animal kindom l Exoskeleton composed mainly of chitin l Distinct parts of the body l Jointed legs and appendages l Bilateral symmetry Classification of arthropods [2] l Trilobites are a group of ancient marine animals l Myriapods comprise millipedes and centipedes l Chelicerates include spiders, mites, scorpions l Hexapods comprise insects l Crustaceans include crabs, lobsters, shrimps and barnacles [1] http: //en. wikipedia. org/wiki/Arthropod/ [2] http: //insected. arizona. edu/arthroinfo. htm http: //evolution. berkeley. edu/evolibrary/images/arthropodphylogeny 7. gif

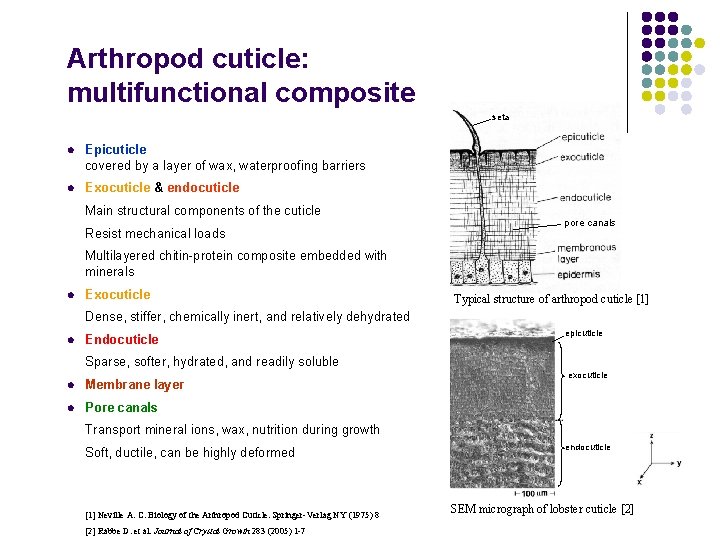
Arthropod cuticle: multifunctional composite seta l Epicuticle covered by a layer of wax, waterproofing barriers l Exocuticle & endocuticle Main structural components of the cuticle Resist mechanical loads pore canals Multilayered chitin-protein composite embedded with minerals l Exocuticle Typical structure of arthropod cuticle [1] Dense, stiffer, chemically inert, and relatively dehydrated l Endocuticle epicuticle Sparse, softer, hydrated, and readily soluble l Membrane layer l Pore canals exocuticle Transport mineral ions, wax, nutrition during growth Soft, ductile, can be highly deformed [1] Neville A. C. Biology of the Arthropod Cuticle. Springer-Verlag NY (1975) 8 [2] Rabbe D. et al. Journal of Crystal Growth 283 (2005) 1 -7 endocuticle SEM micrograph of lobster cuticle [2]

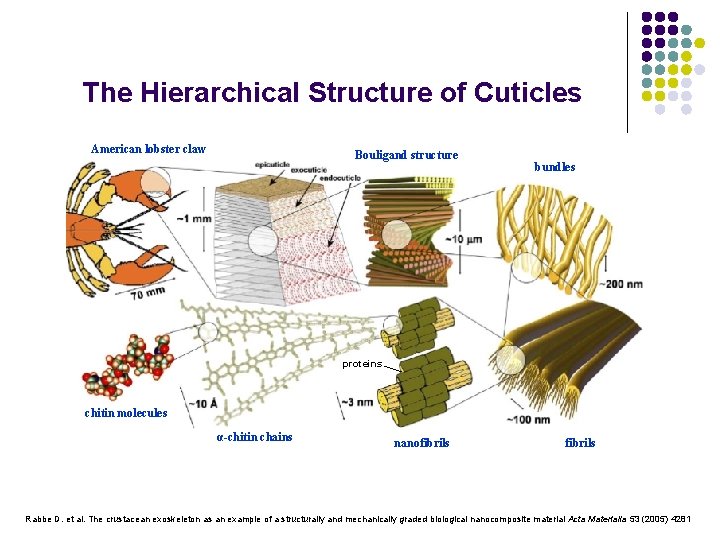
The Hierarchical Structure of Cuticles American lobster claw Bouligand structure bundles proteins chitin molecules α-chitin chains nanofibrils Rabbe D. et al. The crustacean exoskeleton as an example of a structurally and mechanically graded biological nanocomposite material Acta Materialia 53 (2005) 4281

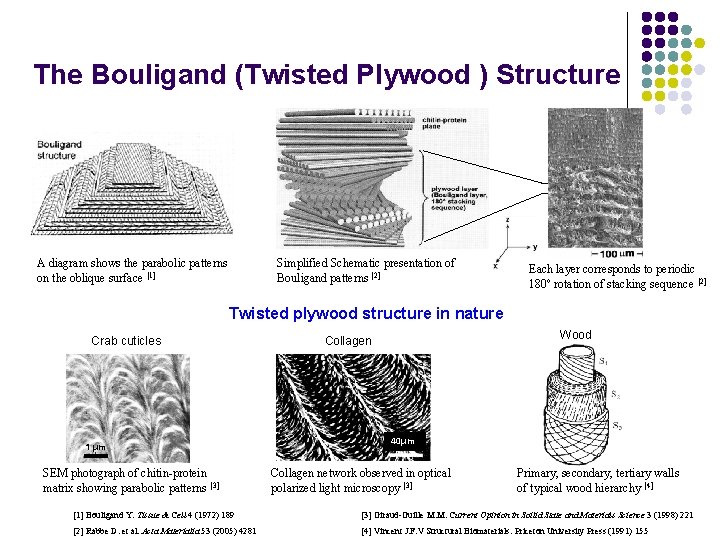
The Bouligand (Twisted Plywood ) Structure A diagram shows the parabolic patterns on the oblique surface [1] Simplified Schematic presentation of Bouligand patterns [2] Each layer corresponds to periodic 180º rotation of stacking sequence [2] Twisted plywood structure in nature Crab cuticles 1 μm SEM photograph of chitin-protein matrix showing parabolic patterns [3] Wood Collagen 40μm Collagen network observed in optical polarized light microscopy [3] Primary, secondary, tertiary walls of typical wood hierarchy [4] [1] Bouligand Y. Tissue & Cell 4 (1972) 189 [3] Giraud-Guille M. M. Current Opinion in Soilid State and Materials Science 3 (1998) 221 [2] Rabbe D. et al. Acta Materialia 53 (2005) 4281 [4] Vincent J. F. V Structural Biomaterials. Priceton University Press (1991) 155

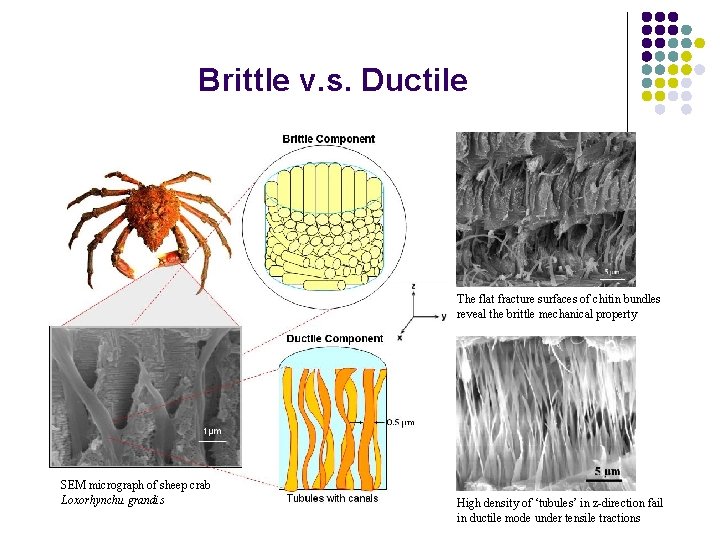
Brittle v. s. Ductile The flat fracture surfaces of chitin bundles reveal the brittle mechanical property SEM micrograph of sheep crab Loxorhynchu grandis High density of ‘tubules’ in z-direction fail in ductile mode under tensile tractions

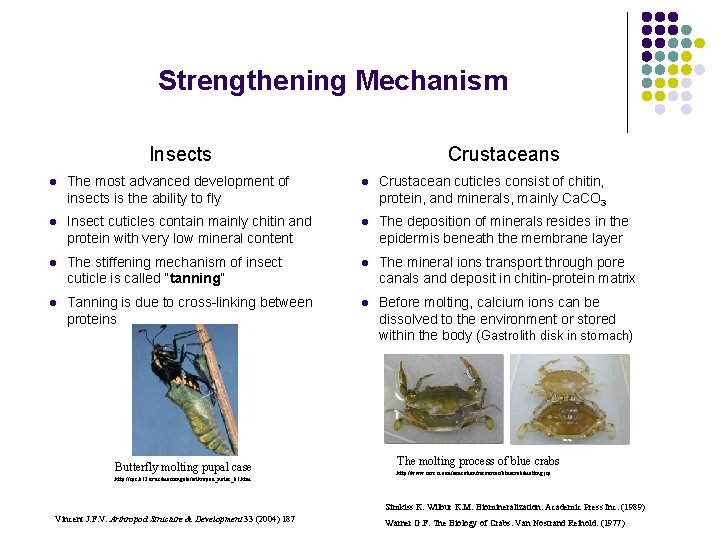
Strengthening Mechanism Insects Crustaceans l The most advanced development of insects is the ability to fly l Crustacean cuticles consist of chitin, protein, and minerals, mainly Ca. CO 3 l Insect cuticles contain mainly chitin and protein with very low mineral content l The deposition of minerals resides in the epidermis beneath the membrane layer l The stiffening mechanism of insect cuticle is called “tanning” l The mineral ions transport through pore canals and deposit in chitin-protein matrix l Tanning is due to cross-linking between proteins l Before molting, calcium ions can be dissolved to the environment or stored within the body (Gastrolith disk in stomach) Butterfly molting pupal case http: //sps. k 12. ar. us/massengale/arthropod_notes_b 1. htm The molting process of blue crabs http: //www. serc. si. edu/education/resources/bluecrab/molting. jsp Simkiss K. Wilbur K. M. Biomineralization. Academic Press Inc. (1989) Vincent J. F. V. Arthropod Structure & Development 33 (2004) 187 Warner G. F. The Biology of Crabs. Van Nostrand Reihold. (1977)

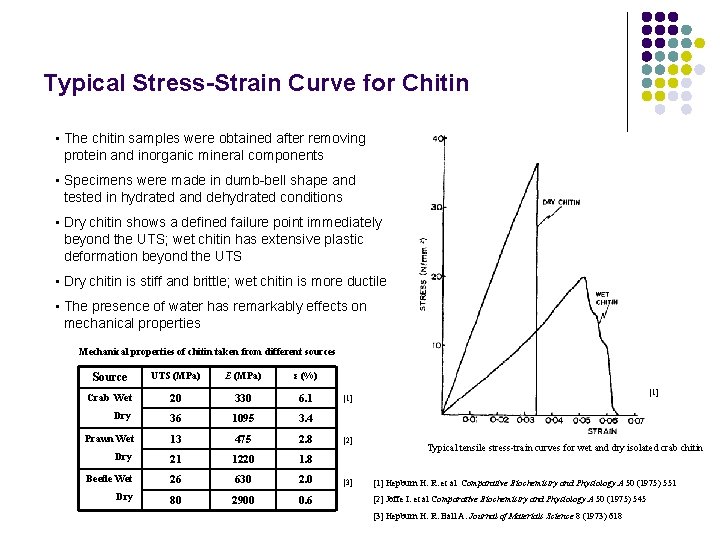
Typical Stress-Strain Curve for Chitin • The chitin samples were obtained after removing protein and inorganic mineral components • Specimens were made in dumb-bell shape and tested in hydrated and dehydrated conditions • Dry chitin shows a defined failure point immediately beyond the UTS; wet chitin has extensive plastic deformation beyond the UTS • Dry chitin is stiff and brittle; wet chitin is more ductile • The presence of water has remarkably effects on mechanical properties Mechanical properties of chitin taken from different sources Source UTS (MPa) E (MPa) ε (%) Crab Wet 20 330 6. 1 Dry 36 1095 3. 4 Prawn Wet 13 475 2. 8 Dry 21 1220 1. 8 Beetle Wet 26 630 2. 0 Dry 80 2900 0. 6 [1] [2] [3] Typical tensile stress-train curves for wet and dry isolated crab chitin [1] Hepburn H. R. et al Comparative Biochemistry and Physiology A 50 (1975) 551 [2] Joffe I. et al Comparative Biochemistry and Physiology A 50 (1975) 545 [3] Hepburn H. R. Ball A. Journal of Materials Science 8 (1973) 618

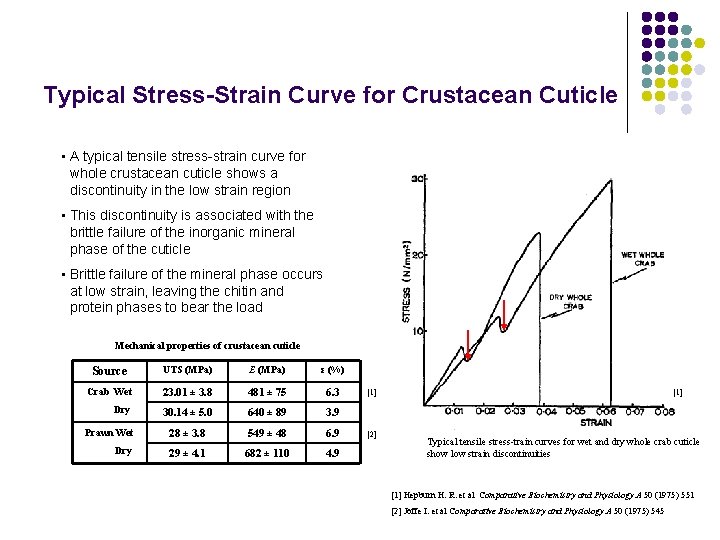
Typical Stress-Strain Curve for Crustacean Cuticle • A typical tensile stress-strain curve for whole crustacean cuticle shows a discontinuity in the low strain region • This discontinuity is associated with the brittle failure of the inorganic mineral phase of the cuticle • Brittle failure of the mineral phase occurs at low strain, leaving the chitin and protein phases to bear the load Mechanical properties of crustacean cuticle Source UTS (MPa) E (MPa) ε (%) Crab Wet 23. 01 ± 3. 8 481 ± 75 6. 3 Dry 30. 14 ± 5. 0 640 ± 89 3. 9 Prawn Wet 28 ± 3. 8 549 ± 48 6. 9 Dry 29 ± 4. 1 682 ± 110 4. 9 [1] [2] [1] Typical tensile stress-train curves for wet and dry whole crab cuticle show low strain discontinuities [1] Hepburn H. R. et al Comparative Biochemistry and Physiology A 50 (1975) 551 [2] Joffe I. et al Comparative Biochemistry and Physiology A 50 (1975) 545

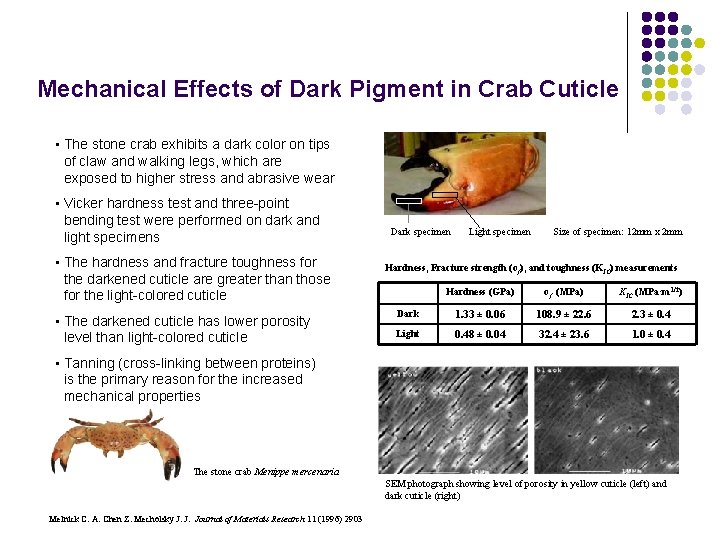
Mechanical Effects of Dark Pigment in Crab Cuticle • The stone crab exhibits a dark color on tips of claw and walking legs, which are exposed to higher stress and abrasive wear • Vicker hardness test and three-point bending test were performed on dark and light specimens • The hardness and fracture toughness for the darkened cuticle are greater than those for the light-colored cuticle • The darkened cuticle has lower porosity level than light-colored cuticle Dark specimen Light specimen Size of specimen: 12 mm x 2 mm Hardness, Fracture strength (σf), and toughness (KIC) measurements Hardness (GPa) σf (MPa) KІC (MPa·m 1/2) Dark 1. 33 ± 0. 06 108. 9 ± 22. 6 2. 3 ± 0. 4 Light 0. 48 ± 0. 04 32. 4 ± 23. 6 1. 0 ± 0. 4 • Tanning (cross-linking between proteins) is the primary reason for the increased mechanical properties The stone crab Menippe mercenaria SEM photograph showing level of porosity in yellow cuticle (left) and dark cuticle (right) Melnick C. A. Chen Z. Mecholsky J. J. Journal of Materials Research 11 (1996) 2903

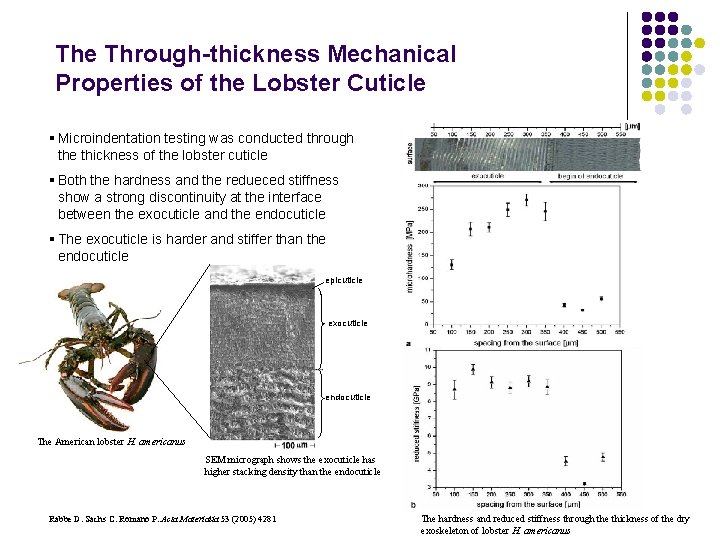
The Through-thickness Mechanical Properties of the Lobster Cuticle § Microindentation testing was conducted through the thickness of the lobster cuticle § Both the hardness and the redueced stiffness show a strong discontinuity at the interface between the exocuticle and the endocuticle § The exocuticle is harder and stiffer than the endocuticle epicuticle exocuticle endocuticle The American lobster H. americanus SEM micrograph shows the exocuticle has higher stacking density than the endocuticle Rabbe D. Sachs C. Romano P. Acta Materialia 53 (2005) 4281 The hardness and reduced stiffness through the thickness of the dry exoskeleton of lobster H. americanus

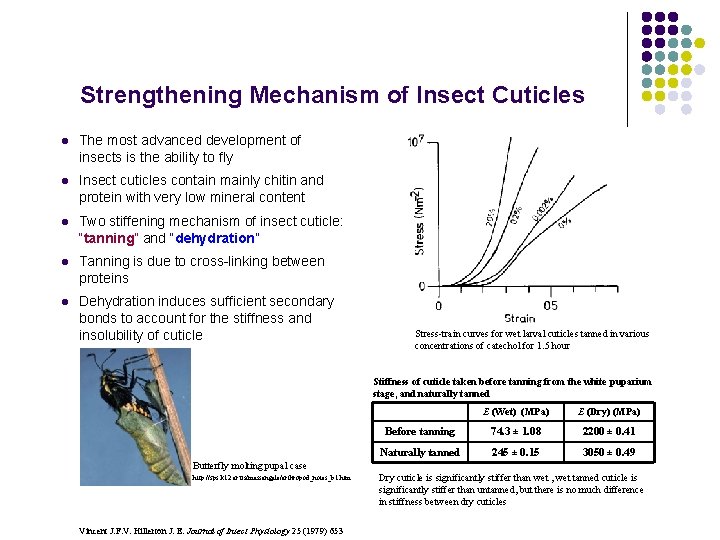
Strengthening Mechanism of Insect Cuticles l The most advanced development of insects is the ability to fly l Insect cuticles contain mainly chitin and protein with very low mineral content l Two stiffening mechanism of insect cuticle: “tanning” and “dehydration” l Tanning is due to cross-linking between proteins l Dehydration induces sufficient secondary bonds to account for the stiffness and insolubility of cuticle Stress-train curves for wet larval cuticles tanned in various concentrations of catechol for 1. 5 hour Stiffness of cuticle taken before tanning from the white puparium stage, and naturally tanned E (Wet) (MPa) E (Dry) (MPa) Before tanning 74. 3 ± 1. 08 2200 ± 0. 41 Naturally tanned 245 ± 0. 15 3050 ± 0. 49 Butterfly molting pupal case http: //sps. k 12. ar. us/massengale/arthropod_notes_b 1. htm Vincent J. F. V. Hillerton J. E. Journal of Insect Physiology 25 (1979) 653 Dry cuticle is significantly stiffer than wet , wet tanned cuticle is significantly stiffer than untanned, but there is no much difference in stiffness between dry cuticles

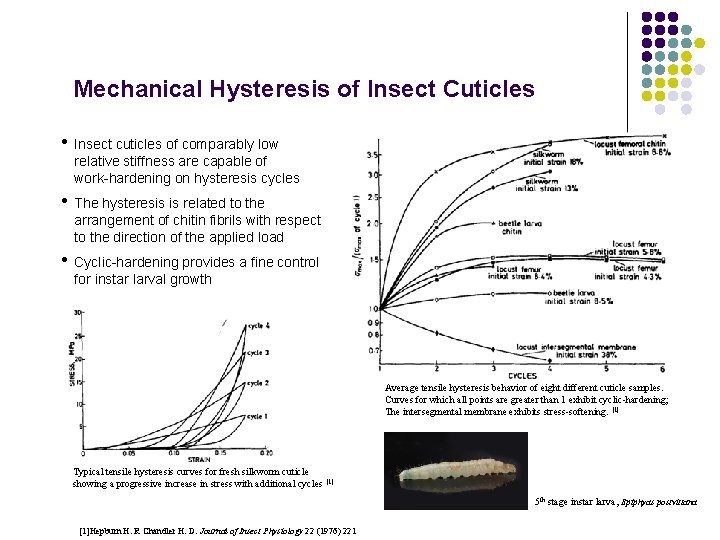
Mechanical Hysteresis of Insect Cuticles • Insect cuticles of comparably low relative stiffness are capable of work-hardening on hysteresis cycles • The hysteresis is related to the arrangement of chitin fibrils with respect to the direction of the applied load • Cyclic-hardening provides a fine control for instar larval growth Average tensile hysteresis behavior of eight different cuticle samples. Curves for which all points are greater than 1 exhibit cyclic-hardening; The intersegmental membrane exhibits stress-softening. [1] Typical tensile hysteresis curves for fresh silkworm cuticle showing a progressive increase in stress with additional cycles [1] 5 th stage instar larva, Epiphyas postvittana [1]Hepburn H. R Chandler H. D. Journal of Insect Physiology 22 (1976) 221

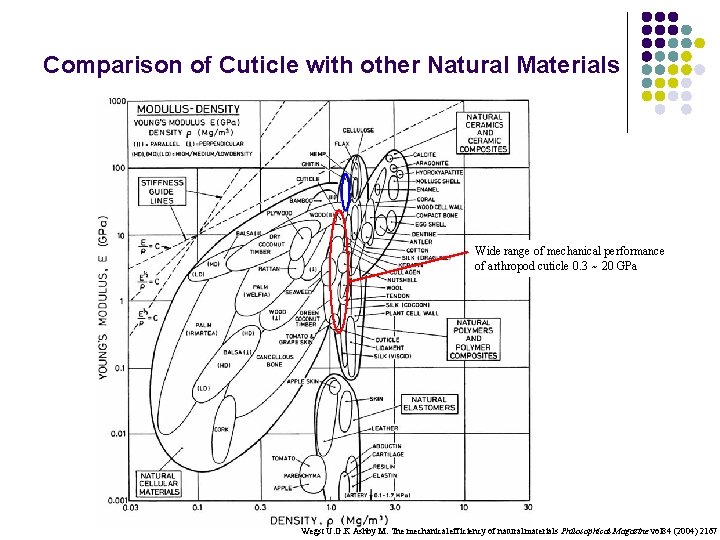
Comparison of Cuticle with other Natural Materials Wide range of mechanical performance of arthropod cuticle 0. 3 ~ 20 GPa Wegst U. G. K Ashby M. The mechanical efficiency of natural materials Philosophical Magazine vol 84 (2004) 2167

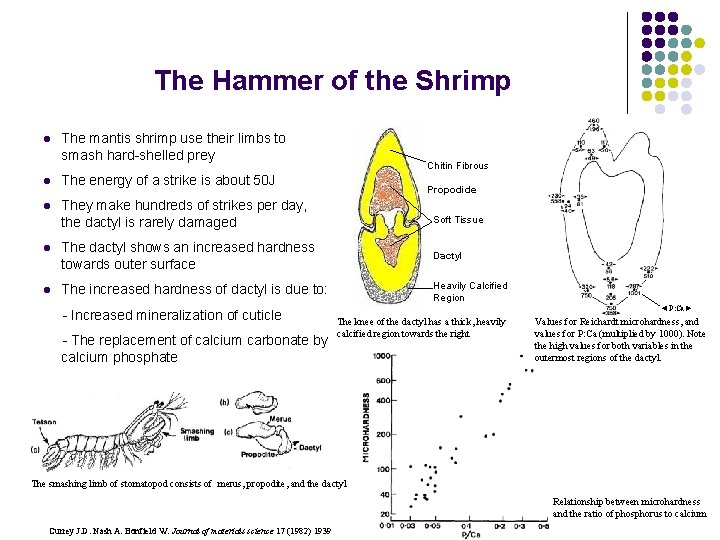
The Hammer of the Shrimp l The mantis shrimp use their limbs to smash hard-shelled prey l The energy of a strike is about 50 J l They make hundreds of strikes per day, the dactyl is rarely damaged Chitin Fibrous Propodide Soft Tissue l The dactyl shows an increased hardness towards outer surface Dactyl l The increased hardness of dactyl is due to: Heavily Calcified Region - Increased mineralization of cuticle - The replacement of calcium carbonate by calcium phosphate ◄P: Ca► The knee of the dactyl has a thick, heavily calcified region towards the right Values for Reichardt microhardness, and values for P: Ca (multiplied by 1000). Note the high values for both variables in the outermost regions of the dactyl. The smashing limb of stomatopod consists of merus, propodite, and the dactyl Relationship between microhardness and the ratio of phosphorus to calcium Currey J. D. Nash A. Bonfield W. Journal of materials science 17 (1982) 1939

Photonic Structures in Nature Underside of wings. When the Morpho lands, it closes its wings showing the brown sides, which eludes their main predators, birds. Blue Morpho butterfly (Morpho menelaus) The brilliant blue butterfly can be found in the rainforests of South America (Brasil & Guyana) Why are their top wings blue? o Blue color is caused by “interference” o Blue light has a wavelength about 400 nm, and is interfered constructively by the slits of the morpho, which range 200 nm apart Why are their underside wings brown? o The underside scales do not cause interference o Brown color are produced by pigment, which is called chemical color Hierarchical structure of the wing ( wing > scales > veins > ridges) Vukusic P. Sambles J. R. Photonic structures in biology Nature 424 (2003) 852 Kertesz K. et al. Photonic crystal strucutres if biological origin Current Applied Physics 6 (2006) 252 -258 Source: http: //webexhibits. org/causesofcolor/15. html

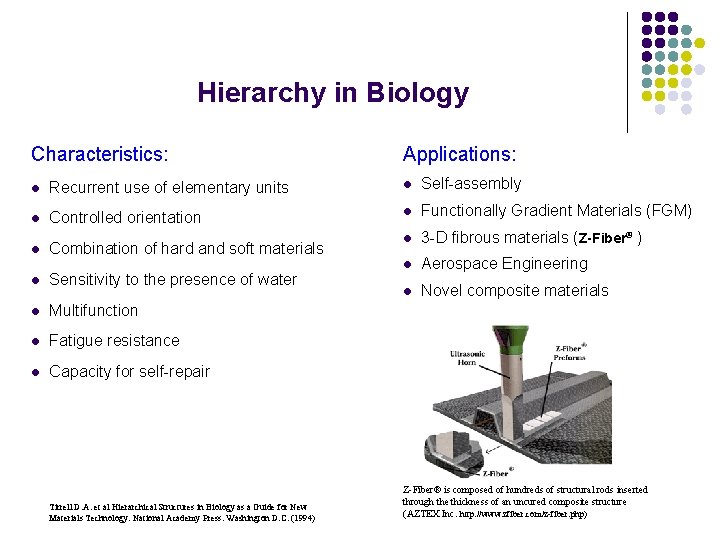
Hierarchy in Biology Characteristics: Applications: l Recurrent use of elementary units l Self-assembly l Controlled orientation l Functionally Gradient Materials (FGM) l 3 -D fibrous materials (Z-Fiber® ) l Aerospace Engineering l Novel composite materials l Combination of hard and soft materials l Sensitivity to the presence of water l Multifunction l Fatigue resistance l Capacity for self-repair Tirrell D. A. et al Hierarchical Structures in Biology as a Guide for New Materials Technology. National Academy Press. Washington D. C. (1994) Z-Fiber® is composed of hundreds of structural rods inserted through the thickness of an uncured composite structure (AZTEX Inc. http: //www. zfiber. com/z-fiber. php)

Conclusions l Chitin and chitosan remain underutilized natural polymers l Chitin and chitosan are promising materials for diverse applications l Isolation and production need to be improved l Hierarchical structures in nature are highly sophisticated, multifunctional, with unique properties Thank you! Questions?
 Chitin
Chitin Chitosan for dogs
Chitosan for dogs Chitin wall
Chitin wall Nature and nature's law lay hid in night
Nature and nature's law lay hid in night Determinace lidské psychiky
Determinace lidské psychiky Vosviewer alternative
Vosviewer alternative Literature review introduction example
Literature review introduction example Types of literature review
Types of literature review Nature review genetics
Nature review genetics Nature review genetics
Nature review genetics Gwas
Gwas Nature review genetics
Nature review genetics Nature review genetics
Nature review genetics Nature review genetics
Nature review genetics The nature of drama perrine's literature
The nature of drama perrine's literature Romanticism period in english literature
Romanticism period in english literature Chapter review motion part a vocabulary review answer key
Chapter review motion part a vocabulary review answer key Writ of certiorari ap gov example
Writ of certiorari ap gov example Nader amin-salehi
Nader amin-salehi What is inclusion and exclusion
What is inclusion and exclusion Narrative review vs systematic review
Narrative review vs systematic review Literature review theory
Literature review theory Literature review
Literature review Review title examples
Review title examples Problem statement literature review example
Problem statement literature review example Literature review gantt chart
Literature review gantt chart Literature review format
Literature review format What is lr
What is lr Literature review on enterprise risk management
Literature review on enterprise risk management Sample of review of literature
Sample of review of literature Synthesis of literature review example
Synthesis of literature review example Importance of literature
Importance of literature Stem literature review example
Stem literature review example Academic poster examples
Academic poster examples Cyberbullying review of related literature
Cyberbullying review of related literature Literature review theory
Literature review theory Literature map template creswell
Literature map template creswell State of the art literature review example
State of the art literature review example



























































