LIQUID CRYSTALS WHAT ARE THEY STRUCTURE OF SOLIDS

- Slides: 8

LIQUID CRYSTALS WHAT ARE THEY?

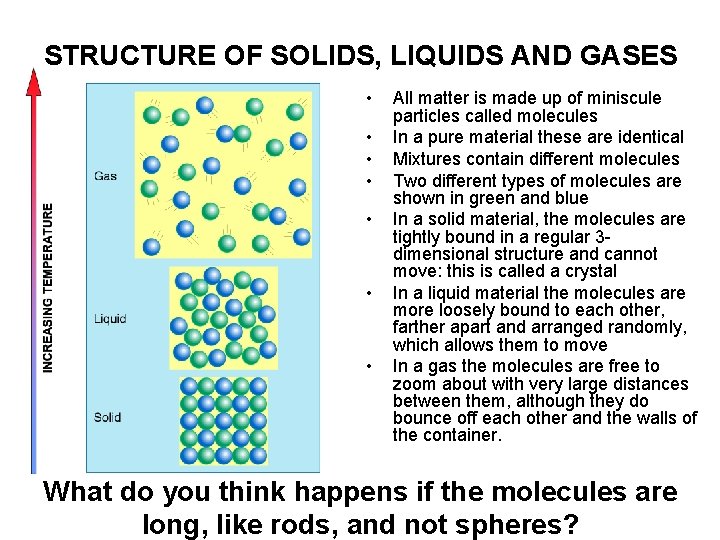
STRUCTURE OF SOLIDS, LIQUIDS AND GASES • • All matter is made up of miniscule particles called molecules In a pure material these are identical Mixtures contain different molecules Two different types of molecules are shown in green and blue In a solid material, the molecules are tightly bound in a regular 3 dimensional structure and cannot move: this is called a crystal In a liquid material the molecules are more loosely bound to each other, farther apart and arranged randomly, which allows them to move In a gas the molecules are free to zoom about with very large distances between them, although they do bounce off each other and the walls of the container. What do you think happens if the molecules are long, like rods, and not spheres?

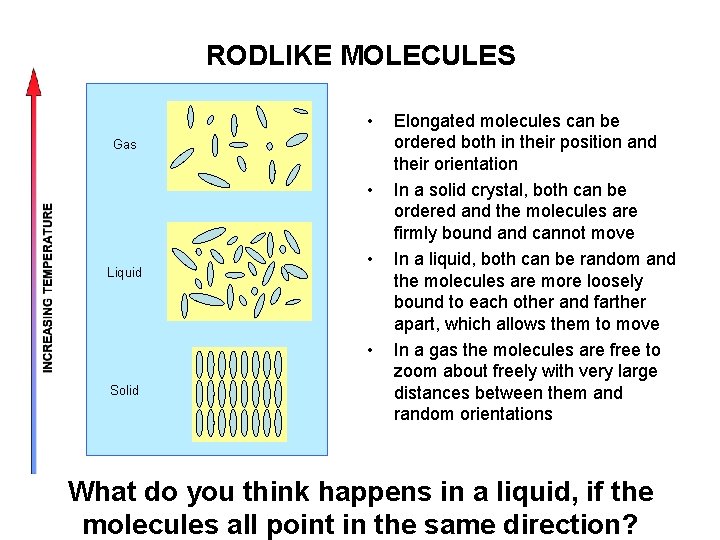
RODLIKE MOLECULES • Gas • Liquid • • Solid Elongated molecules can be ordered both in their position and their orientation In a solid crystal, both can be ordered and the molecules are firmly bound and cannot move In a liquid, both can be random and the molecules are more loosely bound to each other and farther apart, which allows them to move In a gas the molecules are free to zoom about freely with very large distances between them and random orientations What do you think happens in a liquid, if the molecules all point in the same direction?

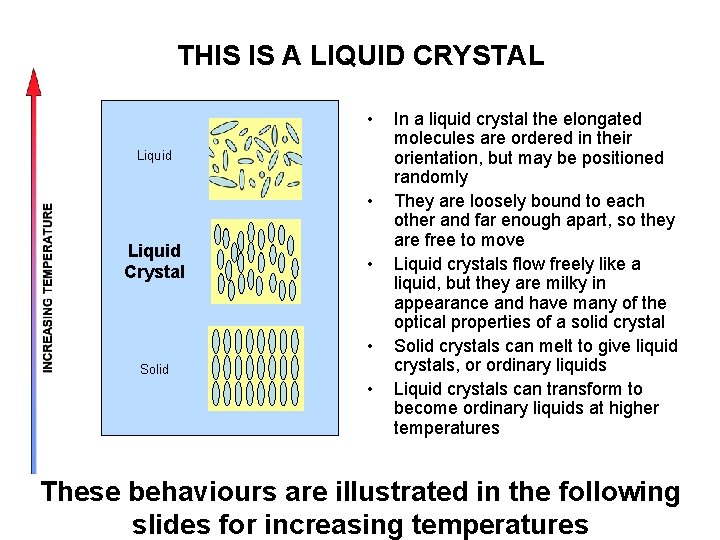
THIS IS A LIQUID CRYSTAL • Liquid Crystal • • Solid • In a liquid crystal the elongated molecules are ordered in their orientation, but may be positioned randomly They are loosely bound to each other and far enough apart, so they are free to move Liquid crystals flow freely like a liquid, but they are milky in appearance and have many of the optical properties of a solid crystal Solid crystals can melt to give liquid crystals, or ordinary liquids Liquid crystals can transform to become ordinary liquids at higher temperatures These behaviours are illustrated in the following slides for increasing temperatures

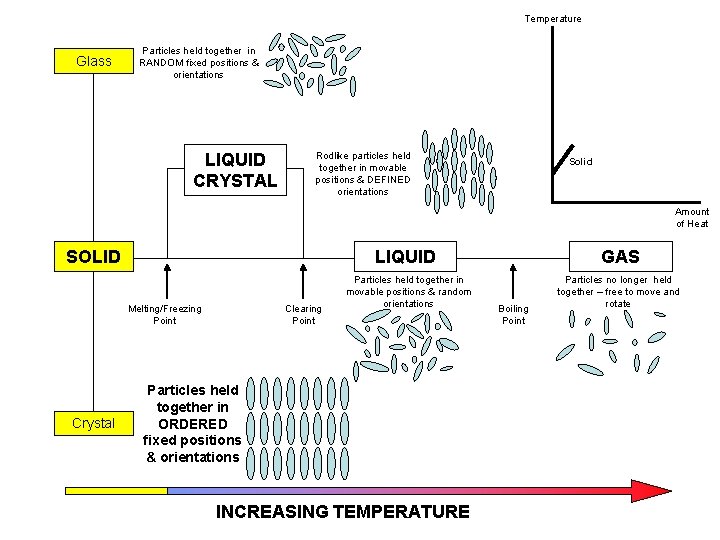
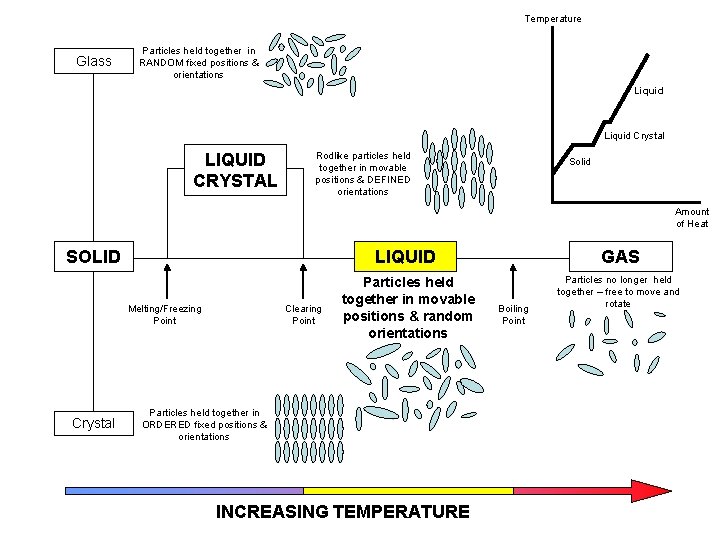
Temperature Glass Particles held together in RANDOM fixed positions & orientations LIQUID CRYSTAL Rodlike particles held together in movable positions & DEFINED orientations Solid Amount of Heat SOLID LIQUID Melting/Freezing Point Crystal Clearing Point Particles held together in movable positions & random orientations Particles held together in ORDERED fixed positions & orientations INCREASING TEMPERATURE GAS Boiling Point Particles no longer held together – free to move and rotate

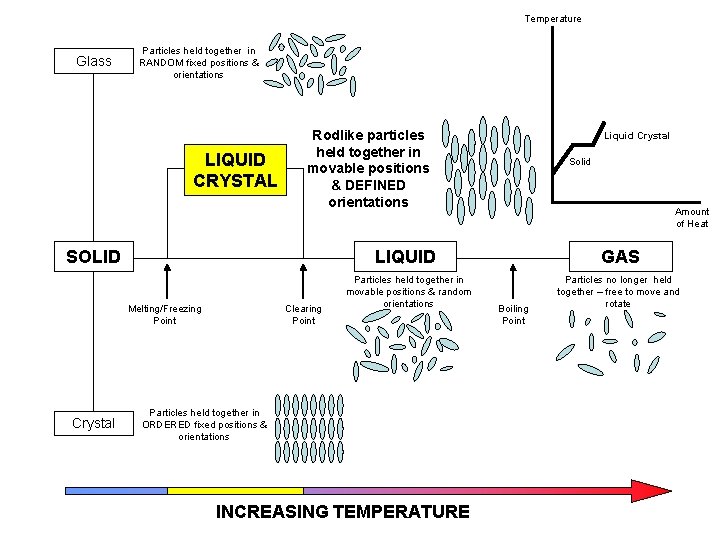
Temperature Glass Particles held together in RANDOM fixed positions & orientations LIQUID CRYSTAL Rodlike particles held together in movable positions & DEFINED orientations SOLID Solid Amount of Heat LIQUID Melting/Freezing Point Crystal Liquid Crystal Clearing Point Particles held together in movable positions & random orientations Particles held together in ORDERED fixed positions & orientations INCREASING TEMPERATURE GAS Boiling Point Particles no longer held together – free to move and rotate

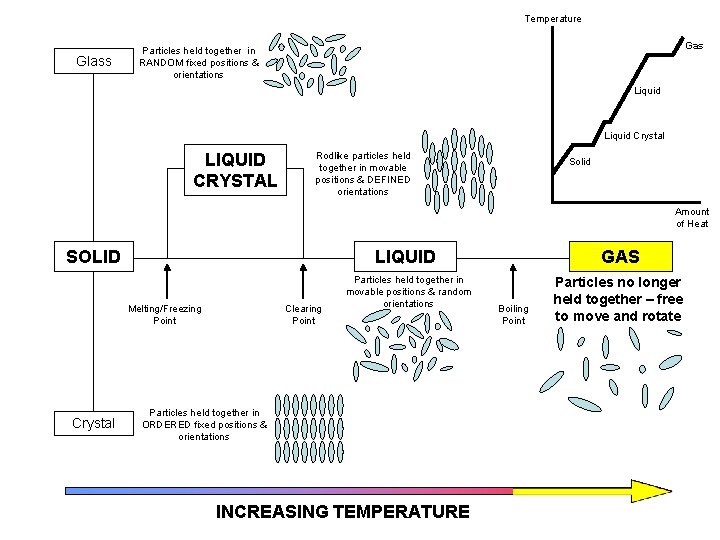
Temperature Glass Particles held together in RANDOM fixed positions & orientations Liquid Crystal LIQUID CRYSTAL Rodlike particles held together in movable positions & DEFINED orientations Solid Amount of Heat SOLID Melting/Freezing Point Crystal Clearing Point LIQUID GAS Particles held together in movable positions & random orientations Particles no longer held together – free to move and rotate Particles held together in ORDERED fixed positions & orientations INCREASING TEMPERATURE Boiling Point

Temperature Glass Gas Particles held together in RANDOM fixed positions & orientations Liquid Crystal LIQUID CRYSTAL Rodlike particles held together in movable positions & DEFINED orientations Solid Amount of Heat SOLID LIQUID Melting/Freezing Point Crystal Clearing Point Particles held together in movable positions & random orientations Particles held together in ORDERED fixed positions & orientations INCREASING TEMPERATURE GAS Boiling Point Particles no longer held together – free to move and rotate