Lessons learnt and challenges ahead GPS REACH conference













- Slides: 21

Lessons learnt and challenges ahead GPS / REACH conference 04 Oct 2011 Athens, Greece L. Heezen Manager Global Chemical Regulations 1

Content • Next deadline 2013 • Working in SIEFs • Industry guidance’s and tools for CSA development and ES communication • REACH legal remedies 2

Next deadline 2013 Early estimates for 2013 registrations: • 3 500 substances – Number of substances revised downwards compared to 2007 estimates – Lessons from 2010 registration: less substances than initially estimated, especially intermediates – Same ratio applied to 2013 estimates • 13 300 new dossiers expected • Market intelligence needed to confirm 3

Working is SIEFs From pre-SIEF to SIEF (2010 experience) • Difficult to form a SIEF and get the work started • Use Substance Identity Profile (SIP) template • Guidance on SIEF formation • SFF not filling in its role • Recommendations to bypass • Companies reluctant to take the LR role • Cefic guidance for LR • Checklist SIEF tasks • Obligations and liabilities of LR • What are intentions of SIEF members? • Use SIEF codes: Leading, Involved, Passive and Dormant • Consortia formation did help • Cefic model REACH SIEF agreement 4

SIEFs: main challenges and learning's Need for an efficient SIEF management process • Early, clear, transparent and regular communication to all SIEF members • Determine substance sameness and communicate to all SIEF members • C&L process • Data availability check • Agreement on LR and notification to ECHA • Cefic REACH model cooperation agreement • Report progress status • Joint/separate CSA/CSR 5

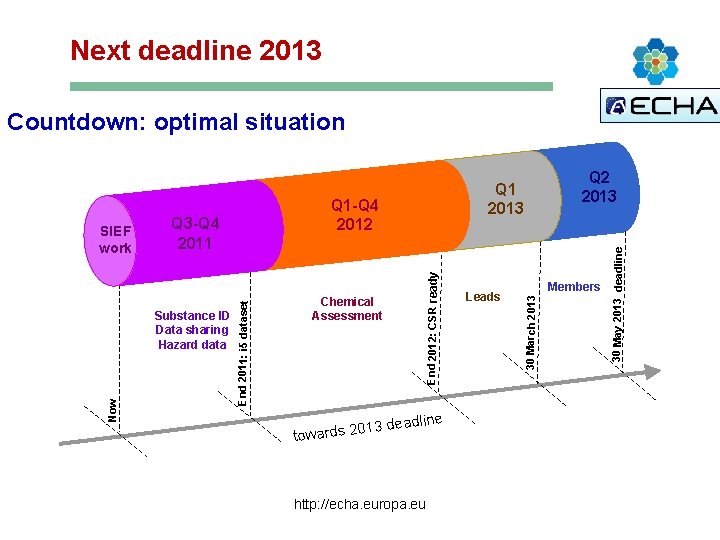
Next deadline 2013 Countdown: optimal situation 3 deadline 01 towards 2 http: //echa. europa. eu Members 30 March 2013 Chemical Assessment Leads 30 May 2013 deadline Q 1 -Q 4 2012 End 2012: CSR ready Substance ID Data sharing Hazard data Now Q 1 2013 2011 End 2011: i 5 dataset SIEF work Start Q 3 -Q 4 CSA Q 2 2013 IUCLID I 5 Z

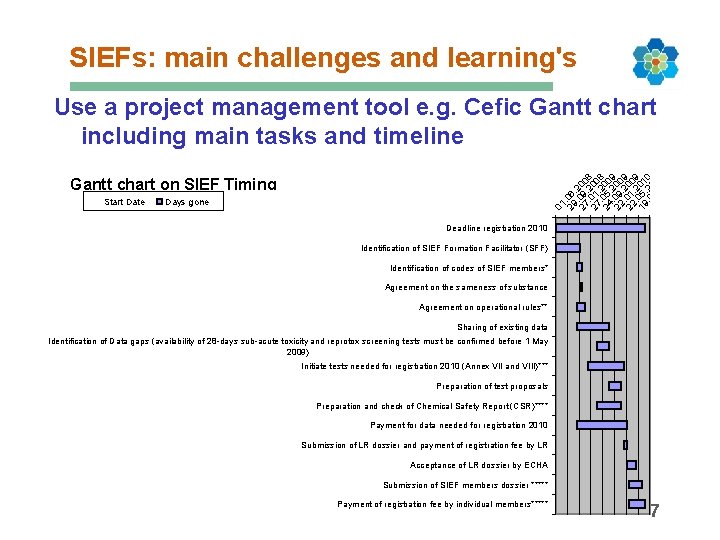
SIEFs: main challenges and learning's Use a project management tool e. g. Cefic Gantt chart including main tasks and timeline Start Date 01 29. 06. 2 27. 09 00. 2 8. 27 01 00. 2 8. 24 05 00. 2 9. 0 22 9 00. 2 9 22. 01 00. 2 9 19. 05 01. 2 0. 0 17 9 01. 0. 20 0 1. 1 20 0 11 Gantt chart on SIEF Timing Days gone Deadline registration 2010 Identification of SIEF Formation Facilitator (SFF) Identification of codes of SIEF members* Agreement on the sameness of substance Agreement on operational rules** Sharing of existing data Identification of Data gaps (availability of 28 -days sub-acute toxicity and reprotox screening tests must be confirmed before 1 May 2009) Initiate tests needed for registration 2010 (Annex VII and VIII)*** Preparation of test proposals Preparation and check of Chemical Safety Report (CSR)**** Payment for data needed for registration 2010 Submission of LR dossier and payment of registration fee by LR Acceptance of LR dossier by ECHA Submission of SIEF members dossier ***** Payment of registration fee by individual members***** 7

SIEF: main challenges and learning’s Consistent and efficient communication • SIEF Management can be done by LR or outsourced to service providers e. g. Reach. Centrum • SIEF communication platform is key e. g. : LINKin. SIEF - New tool based on experience made during the previous phase - Focus on practicability and user friendliness - For LR: Flexible surveys and centralized document storage. Support for compliance/liabilities - For SIEF members: Free; one single point for access SIEF information in a simple & secured way 8

SIEF: main challenges and learning’s Cost sharing • Should be fair, transparent and non-discriminatory • Pay for the data you need(tonnage band, intermediate…) • Explanation of cost sharing system e. g. coverletter + early estimate if possible • When will SIEF members get the data? Lo. A procedure • Costs may include: - SIEF management - Preparation of IUCLID dossier, RSS, CSR - Generation of invoices, letters of access, etc. - Provision for reimbursement (with a threshold? ) Consult Cefic notes on cost sharing 9

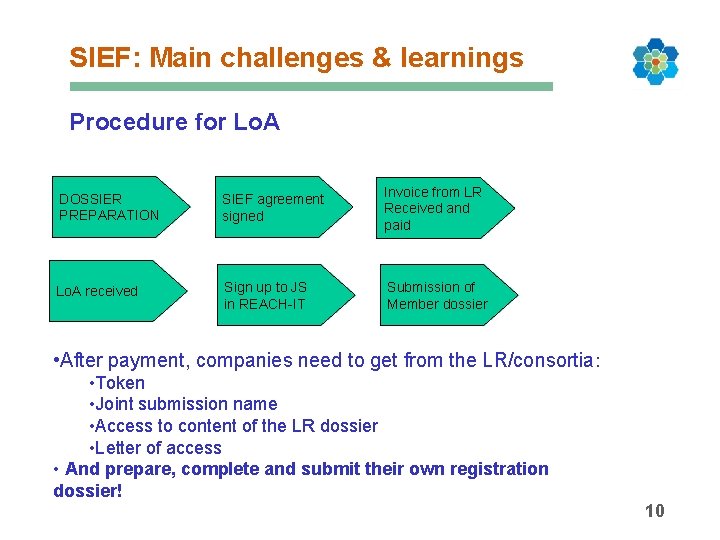
SIEF: Main challenges & learnings Procedure for Lo. A DOSSIER PREPARATION SIEF agreement signed Invoice from LR Received and paid Lo. A received Sign up to JS in REACH-IT Submission of Member dossier • After payment, companies need to get from the LR/consortia: • Token • Joint submission name • Access to content of the LR dossier • Letter of access • And prepare, complete and submit their own registration dossier! 10

SIEF: main challenges and learning’s Handling new registrants in SIEF’s • SIEF that have submitted dossier in 2010: • LR sends out note with procedure to follow and timing to SIEF members • LR to assure the latest SIEF membership from REACH IT • Transparent SIEF communication including • clear cost sharing rules • Scope of LR dossier (CSR jointly prepared? ) • SIEF agreement remains key! • Model agreement on Cefic web 11

2013 registration: challenges New SIEFs for 2013 will look different: ü Expect fewer substances will be handled in consortia ü Smaller companies will require registrations ü less experienced? ü less resources? ü SIEF management challenges will become even more important ü Substances are likely to be less data rich ü Average cost per dossier could go up ü Less experienced Lead Registrants to manage SIEFs 12

2013 registration: challenges New registrants: ü Check if substance has been registered and contact LR/consortium ü If not registered yet, consider forming SIEF leadership team ü If needed, do careful selection of service provider ü Do pre-SIEF steps as soon as possible. Don’t loose time with SIP and agreement ü Use documents and tools on Cefic website http: //www. cefic. org/Industry-support/Implementing-reach/ ü Use best practices and tools, don’t invent the wheel again! 13

Update of dossiers When to update a dossier? What is „without undue delay‟ (Art 22) ? • Updates need time! - E. g. preparation of CSR may take more than one year! - Realistic time for companies • Case by case: - How foreseeable the change was Difficulty of the update Interim measures may be needed Change of classification: if R 50/53 companies should not wait for 2013

Dissemination of information from registration dossiers: – Some information already available on ECHA website (LR + individual) – Publication of Company names + details in the future – Disseminated information cannot be used for free – C&L inventory to be published by the end of the summer Check your dossiers to avoid publication of confidential information!

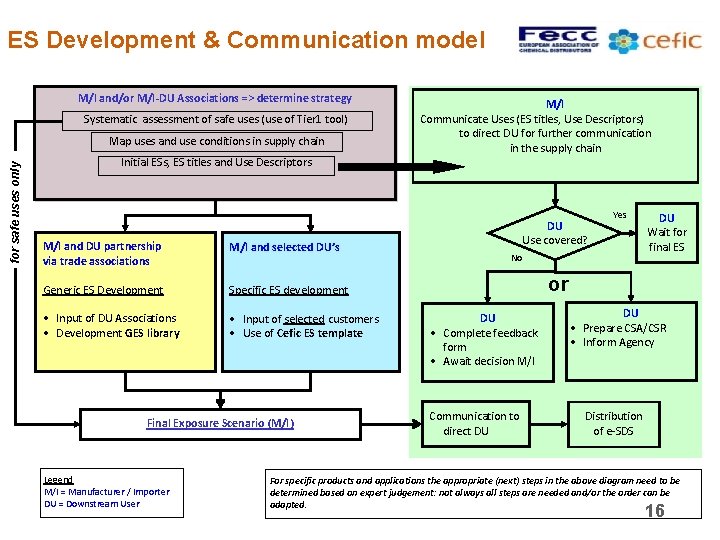
ES Development & Communication model M/I and/or M/I-DU Associations => determine strategy Systematic assessment of safe uses (use of Tier 1 tool) for safe uses only Map uses and use conditions in supply chain M/I Communicate Uses (ES titles, Use Descriptors) to direct DU for further communication in the supply chain Initial ESs, ES titles and Use Descriptors M/I and DU partnership via trade associations M/I and selected DU’s Generic ES Development Specific ES development • Input of DU Associations • Development GES library • Input of selected customers • Use of Cefic ES template Final Exposure Scenario (M/I) Legend M/I = Manufacturer / Importer DU = Downstream User DU Use covered? Yes No DU Wait for final ES or DU • Complete feedback form • Await decision M/I Communication to direct DU DU • Prepare CSA/CSR • Inform Agency Distribution of e-SDS For specific products and applications the appropriate (next) steps in the above diagram need to be determined based on expert judgement: not always all steps are needed and/or the order can be adapted. 16

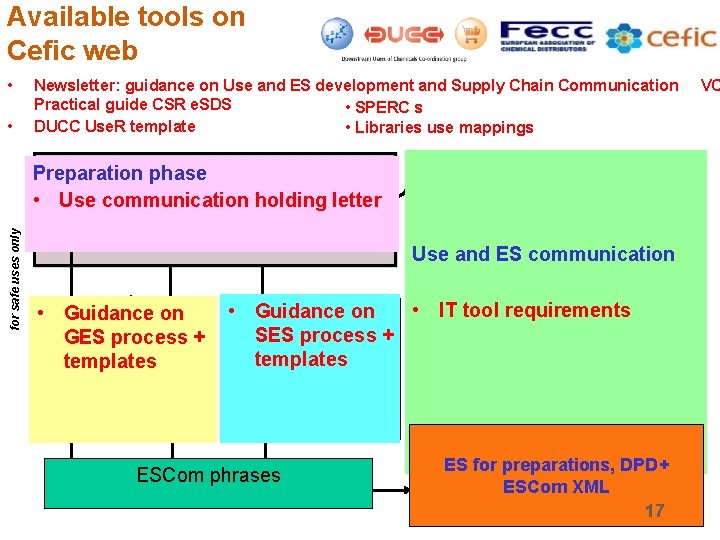
Available tools on Cefic web • • Newsletter: guidance on Use and ES development and Supply Chain Communication Practical guide CSR e. SDS • SPERC s DUCC Use. R template • Libraries use mappings M/I and/or M/I-DU Associations => determine strategy for safe uses only Preparation phase Systematic assessment of safe uses (use of Tier 1 tool) • Use communication holding Map uses and use conditions in supply chain letter Initial ESs, ES titles and Use Descriptors Use and ES communication and DU partnershipon • M/I Guidance via trade associations GES process + Generic ES Development templates • Input of DU Associations • Development GES library DU Use covered? Yes • IT tool requirements selected DU’s on • M/I and Guidance No SES process + or Specific ES development templates • Input of selected customers • Use of Cefic ES template ESCom phrases Final Exposure Scenario (M/I) DU • Complete feedback form • Await decision M/I DU Wait for final ES DU • Prepare CSA/CSR • Inform Agency ES for preparations, DPD+ Communication to Distribution ESCom XML direct DU of e-SDS 17 VC

REACH legal remedies 18

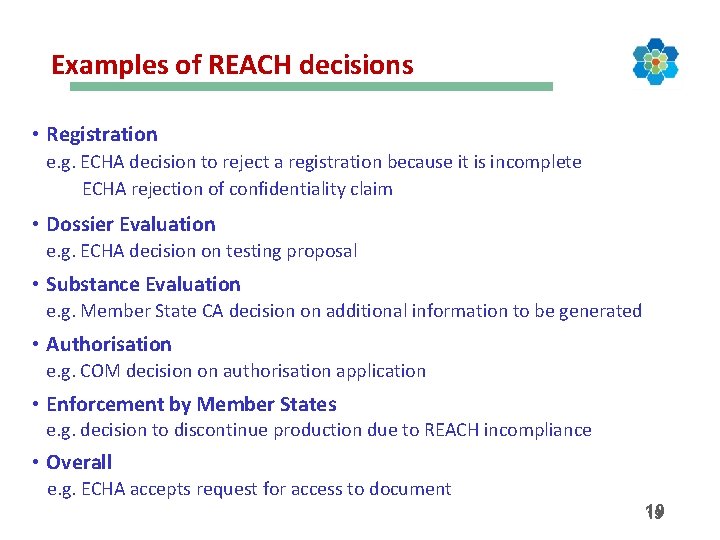
Examples of REACH decisions • Registration e. g. ECHA decision to reject a registration because it is incomplete ECHA rejection of confidentiality claim • Dossier Evaluation e. g. ECHA decision on testing proposal • Substance Evaluation e. g. Member State CA decision on additional information to be generated • Authorisation e. g. COM decision on authorisation application • Enforcement by Member States e. g. decision to discontinue production due to REACH incompliance • Overall e. g. ECHA accepts request for access to document 19 19

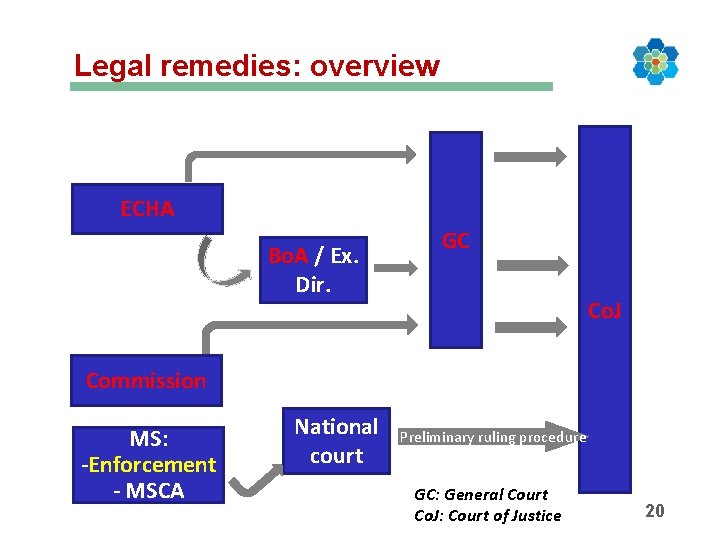
Legal remedies: overview ECHA Bo. A / Ex. Dir. GC Co. J Commission MS: -Enforcement - MSCA National court Preliminary ruling procedure GC: General Court Co. J: Court of Justice 20

Cefic industry support: http: //www. cefic. org/Industry-support/Implementing-reach/Documents-and-Tools 1/ Thank You! lhe@cefic. be 21