Horizon Scanning Why how and what lies ahead


















- Slides: 33

Horizon Scanning Why, how and what lies ahead? Produced to support the Prescribing Outlook series October 2012 Helen Davis, North West Medicines Information Centre

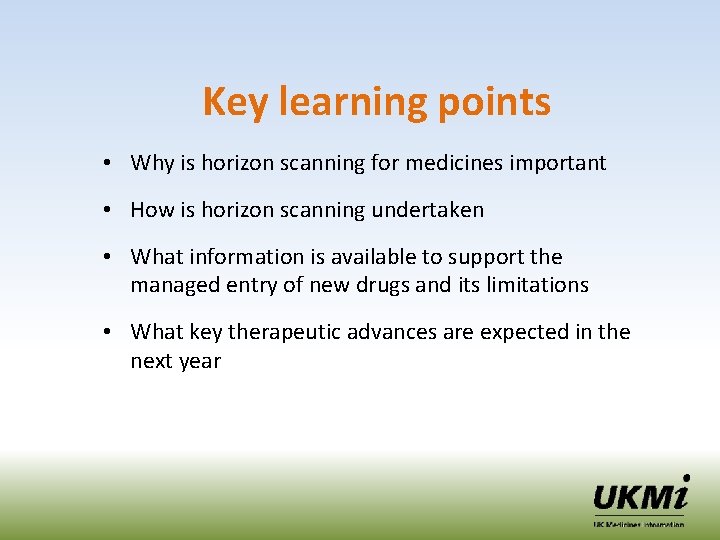
Key learning points • Why is horizon scanning for medicines important • How is horizon scanning undertaken • What information is available to support the managed entry of new drugs and its limitations • What key therapeutic advances are expected in the next year

What is horizon scanning? Horizon Scanning has been defined as: ‘the systematic examination of potential threats, opportunities and likely future developments……. ' Horizon scanning for medicines aims to identify: • treatments likely to become available to the NHS that may have significant implications for – clinical practice – service design – finance • potential disinvestments

Why horizon scan for medicines? Informs and primes providers and commissioners to proactively implement management strategies – – – Anticipate pressures (financial and service delivery) Manage budgets Plan services - new and redesign Identify areas for disinvestment Manage entry into hospital/ formulary /practice etc Identify drugs suitable for homecare

A woman with advanced kidney cancer and six months left to live says she is missing out on a potentially life-saving drug…….

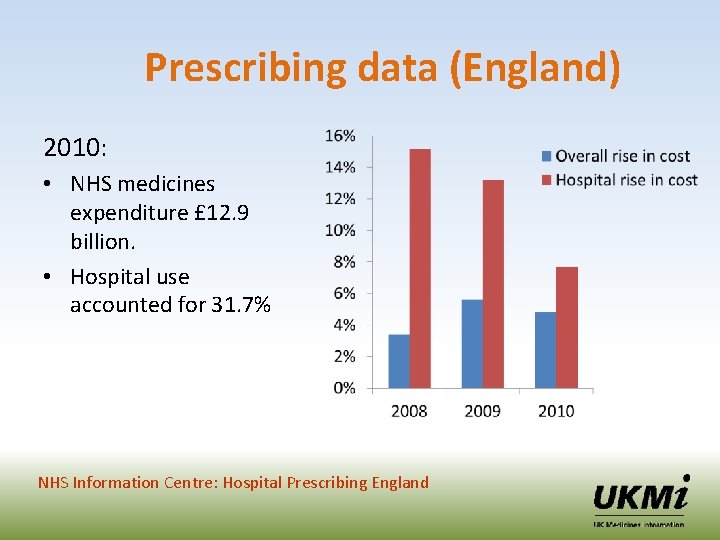
Prescribing data (England) 2010: • NHS medicines expenditure £ 12. 9 billion. • Hospital use accounted for 31. 7% NHS Information Centre: Hospital Prescribing England

Drivers of growth in prescribing • New drugs for diseases where previous options were limited e. g. rare genetic diseases • Expanded indications (increase in eligible population) e. g. chemotherapy drugs • New drug regimens or maintenance treatments added to standard therapy e. g. chemotherapy, antidiabetes • Displacement of old drugs with new drugs at higher cost e. g. “biologicals”, oral anticoagulants • ‘Medicalisation’ e. g. social anxiety • Ageing population

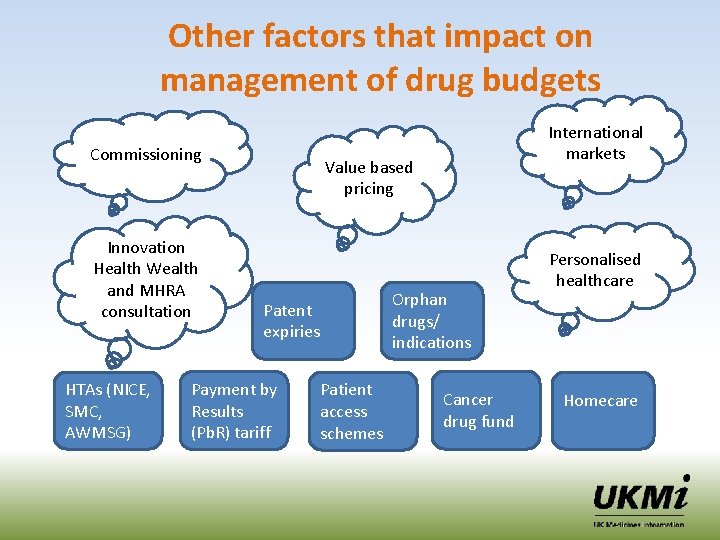
Other factors that impact on management of drug budgets Commissioning Innovation Health Wealth and MHRA consultation HTAs (NICE, SMC, AWMSG) International markets Value based pricing Patent expiries Payment by Results (Pb. R) tariff Patient access schemes Orphan drugs/ indications Cancer drug fund Personalised healthcare Homecare

Information sources used by horizon scanners • Specialist media for press releases highlighting – conference presentations – dates for submission to licensing authorities – plans for development • Specialist websites, databases and journals – UK Pharma. Scan • Industry (contacts, websites, annual reports) • Licensing agencies • Clinical trial registries • Clinical specialists • Other horizon scanners

UK Horizon scanning organisations Health & Social Care Northern Ireland Medicines and Prescribing centre

How does the UKMi horizon scanning process work? Systematic early identification (horizon scanning) Filtration and selection Information retrieval Prioritisation Assessment Dissemination

UKMi Horizon scanning products Prescribing Outlook www. nelm. nhs. uk Password restricted to NHS

Horizon scanning challenges • Licence extensions/ new formulations are difficult to track and time frames for approval are shorter • Company acquisitions vs. in-house R&D (biotech) • Company mergers • Confidentiality issues • Epidemiology or target population may be difficult to define and quantify

Horizon scanning challenges • Regulatory delays • Differences in views between licensing authorities • Regulatory transparency differences • Indication applied for may not be the same as that eventually approved • Cost is rarely known prior to launch • Rate and extent of market uptake is difficult to forecast

Factors influencing impact Drug specific Anticipated licence? Formulation and administration? First in class? Place in therapy? Significant improvement in disease management? Other trials ongoing? (Licence extensions are easier to obtain and there may be off label use. ) • Cost of drug, administration and testing • What could be its USP (unique selling point)? • • •

Factors influencing impact External factors • Size of target population i. e. large population or significant subset of large population? What is large? • Will it change where patients are treated e. g. hospital vs. intermediate vs. home vs. primary care? • Local use (in ongoing clinical trials or unlicensed use)? • Funding of services? E. g National commissioning • Where in NICE agenda? • Which company? • Media/public interest?

Factors UKMi use for prioritisation • • • significant improvement in disease management? additional therapy or displacement of existing therapies? first in class or has a major new indication? limited other drug/non-drug alternatives? high cost? service implications e. g. route/ formulation/ method of delivery the drug or disease area is considered an NHS priority in the EU licensing process significant additional indications in the advanced pipeline stage likely to be significant media interest.

Who is involved in UKMi prioritisation? • UKMi pharmacists with extensive horizon scanning expertise • Primary care/ commissioning pharmacists • Secondary care/ interface pharmacists • Other people/organisations with horizon scanning expertise

UKMi Horizon scanning products New Drugs Online (NDO) database • Accessible via NHS Evidence (limited) and UKMi (full access for NHS staff) websites • Contains over 1300 active monographs • Updated daily • In September 2012 – 356 monographs updated – 14 evidence based evaluations added – 2, 833 registered users • Monthly newsletter sent to registered users (NHS only) • Reporting facility (NHS only)

NDO content Name (generic, company, synonym) Indication, formulation Pharmacology, epidemiology Key trial data Stage in licensing process (EU, US), anticipated UK launch date • Orphan status • Links to independent evaluations e. g. NHSC, LNDG • In NICE pipeline • • •

Other publications relevant to medicines budget planning • • • NICE/ SMC/ AWMSG guidance London New Drugs Group reviews UKMi Prescribing Outlook cost calculator UKMi New Medicines Profiles UKMi IFR summaries UKMi NICE bites MPC* Evidence summaries: new medicines Forward Look (Scotland) Regional advisory committees e. g. NETAG, MTRAC * Medicines Prescribing Centre (formerly NPC – National Prescribing Centre)

2012/13 Key pressures due to new medicines or licence extensions

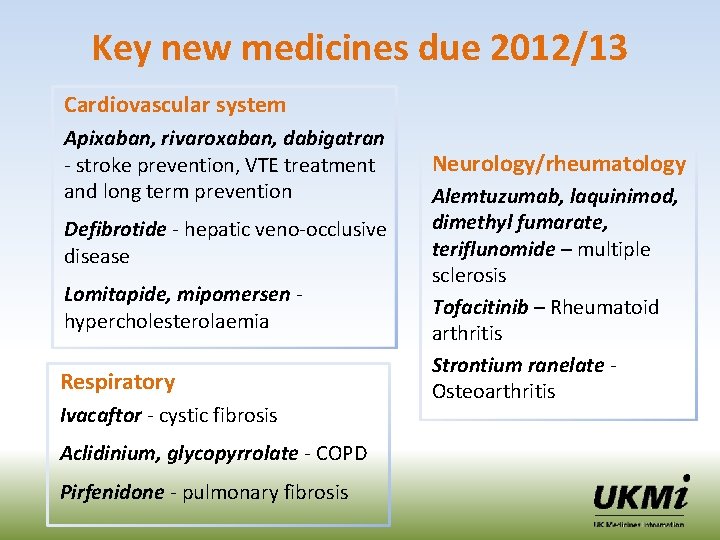
Key new medicines due 2012/13 Cardiovascular system Apixaban, rivaroxaban, dabigatran - stroke prevention, VTE treatment and long term prevention Defibrotide - hepatic veno-occlusive disease Lomitapide, mipomersen hypercholesterolaemia Respiratory Ivacaftor - cystic fibrosis Aclidinium, glycopyrrolate - COPD Pirfenidone - pulmonary fibrosis Neurology/rheumatology Alemtuzumab, laquinimod, dimethyl fumarate, teriflunomide – multiple sclerosis Tofacitinib – Rheumatoid arthritis Strontium ranelate Osteoarthritis

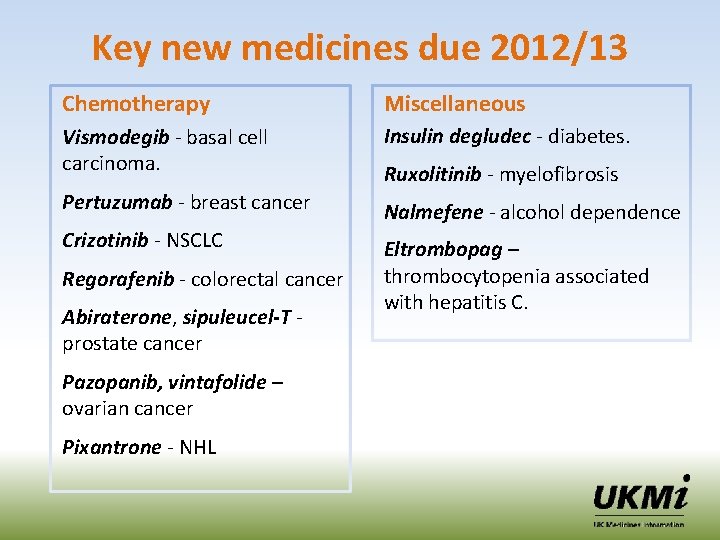
Key new medicines due 2012/13 Chemotherapy Miscellaneous Vismodegib - basal cell carcinoma. Insulin degludec - diabetes. Pertuzumab - breast cancer Nalmefene - alcohol dependence Crizotinib - NSCLC Eltrombopag – thrombocytopenia associated with hepatitis C. Regorafenib - colorectal cancer Abiraterone, sipuleucel-T prostate cancer Pazopanib, vintafolide – ovarian cancer Pixantrone - NHL Ruxolitinib - myelofibrosis

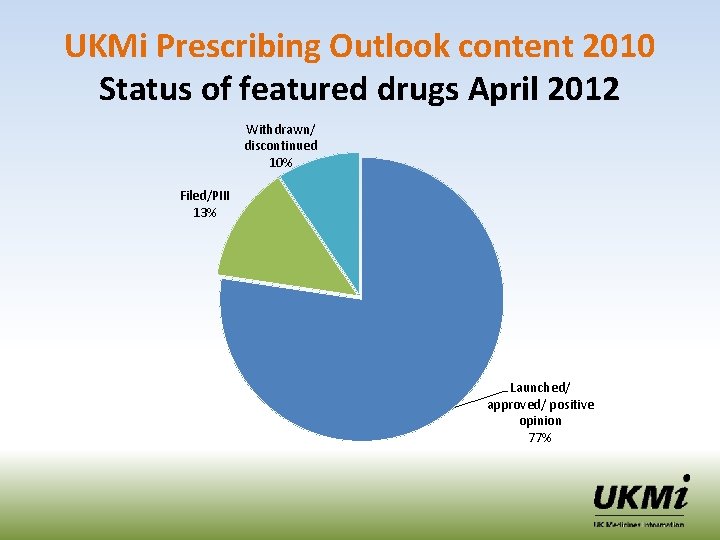
UKMi Prescribing Outlook content 2010 Status of featured drugs April 2012 Withdrawn/ discontinued 10% Filed/PIII 13% Launched/ approved/ positive opinion 77%

Reasons for delay • Licensing process raises questions causing – delay – withdrawal from licensing process – discontinuation • Once licensed the company may not launch in the UK at all • Waiting for NICE appraisal/ reimbursement negotiations

What happens when managed entry is not planned?

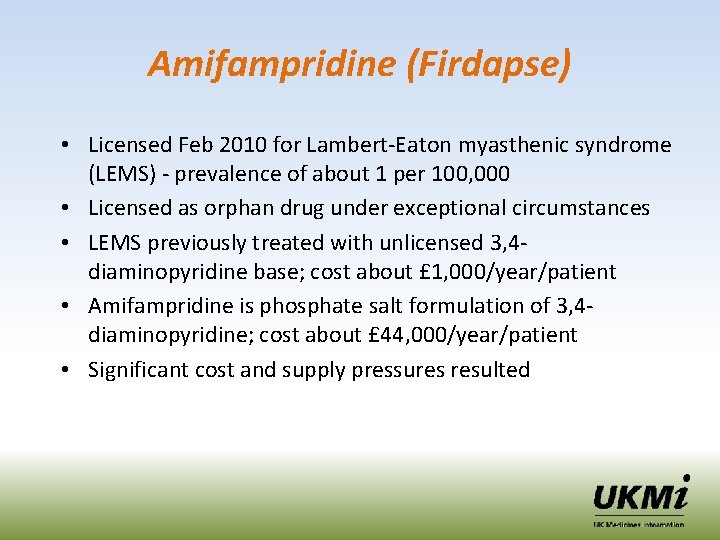
Amifampridine (Firdapse) • Licensed Feb 2010 for Lambert-Eaton myasthenic syndrome (LEMS) - prevalence of about 1 per 100, 000 • Licensed as orphan drug under exceptional circumstances • LEMS previously treated with unlicensed 3, 4 diaminopyridine base; cost about £ 1, 000/year/patient • Amifampridine is phosphate salt formulation of 3, 4 diaminopyridine; cost about £ 44, 000/year/patient • Significant cost and supply pressures resulted

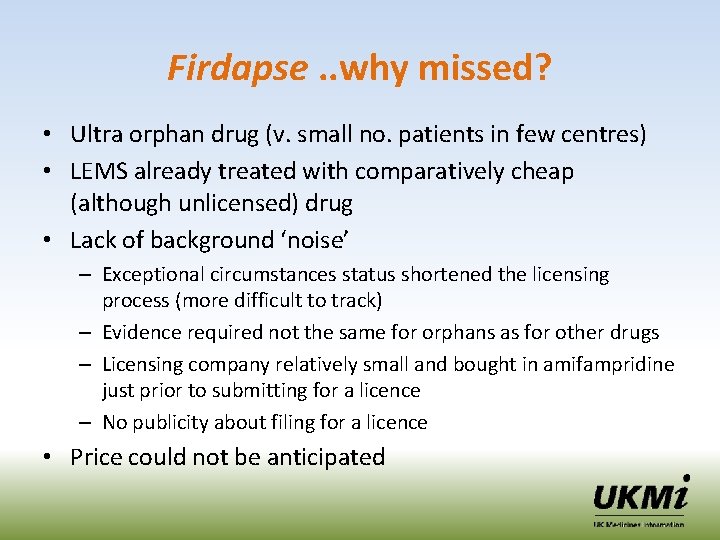
Firdapse. . why missed? • Ultra orphan drug (v. small no. patients in few centres) • LEMS already treated with comparatively cheap (although unlicensed) drug • Lack of background ‘noise’ – Exceptional circumstances status shortened the licensing process (more difficult to track) – Evidence required not the same for orphans as for other drugs – Licensing company relatively small and bought in amifampridine just prior to submitting for a licence – No publicity about filing for a licence • Price could not be anticipated

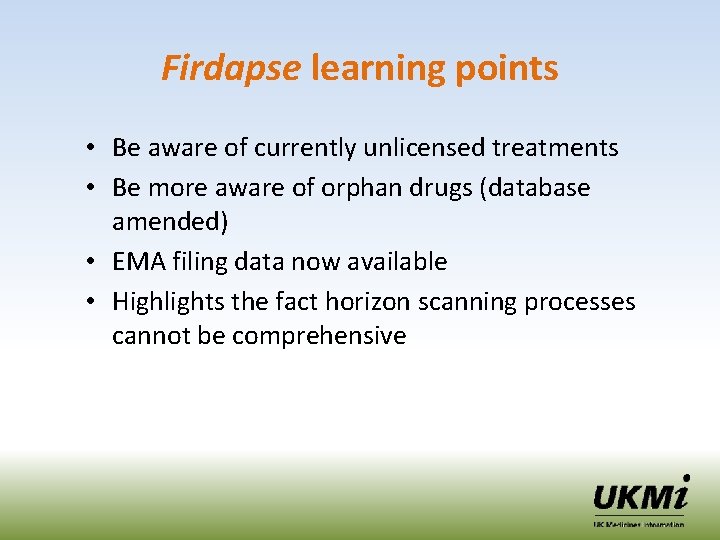
Firdapse learning points • Be aware of currently unlicensed treatments • Be more aware of orphan drugs (database amended) • EMA filing data now available • Highlights the fact horizon scanning processes cannot be comprehensive

What can clinical pharmacists do? • Utilise horizon scanning resources • Be aware of developments within your speciality • Liaise with key clinicians and budget holders to raise awareness and ensure new medicines and licence extensions are planned for

What can clinical pharmacists do? • Understand NHS funding mechanisms and service issues • Highlight key developments to horizon scanners

Thank you Any questions?
 Scan the horizon to see what lies ahead
Scan the horizon to see what lies ahead Horizon-scanning
Horizon-scanning Delta scanning centre
Delta scanning centre Why why why why
Why why why why Dont ask why why why
Dont ask why why why The difference between right and wrong is clear
The difference between right and wrong is clear Damned lies and statistics summary
Damned lies and statistics summary Great gatsby one pager
Great gatsby one pager Proverbs 25 nkjv
Proverbs 25 nkjv Turn left turn right go straight on
Turn left turn right go straight on Planning is looking ahead and control is
Planning is looking ahead and control is Go straight ahead and turn right
Go straight ahead and turn right Soil health
Soil health The camera never lies answers
The camera never lies answers Lays vs lies
Lays vs lies Satan the father of lies
Satan the father of lies 適配度指標
適配度指標 Satan the father of lies niv
Satan the father of lies niv Lies allard
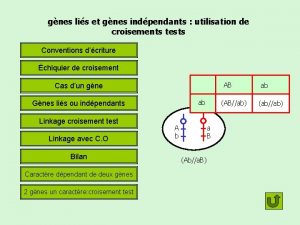
Lies allard échiquier de croisement gènes liés
échiquier de croisement gènes liés Pinch off voltage
Pinch off voltage Deep asleep deep asleep it lies
Deep asleep deep asleep it lies There are three kinds of lies
There are three kinds of lies India lies in which hemisphere
India lies in which hemisphere A deep valley with very steep sides
A deep valley with very steep sides Urogenital triangle boundaries
Urogenital triangle boundaries Lay in a sentence
Lay in a sentence Here lies prosperity cartoon
Here lies prosperity cartoon Qualitative free body diagram
Qualitative free body diagram Adler birth order
Adler birth order The dante project synopsis
The dante project synopsis Radiohead just lyrics
Radiohead just lyrics Thick underground stem that lies horizontally
Thick underground stem that lies horizontally Sir philip sidney an apology for poetry analysis
Sir philip sidney an apology for poetry analysis