Lecture Forensic Toxicology Poisons Alcohol Toxicology is defined
















- Slides: 21

Lecture: Forensic Toxicology Poisons & Alcohol Toxicology is defined as the study of the adverse effects of chemicals on living organisms. Forensic toxicology is defined as the application of toxicology for the purposes of the law. ØPostmortem forensic toxicology. ØHuman performance toxicology. ØForensic drug testing.

History N Ancient Egyptians and Grecians reported poisonings due to herbs, plants and food. N Opium, arsenic and hydrocyanic acid were used throughout Europe during the middle ages. N Philippus Theophrastus Aureolus Bombastus von Hohenheim (or Paracelsus) observed that any substance could be a poison, depending on its dose N “ What is there that is not poison? All things are poison and nothing without poison. Solely the dose determines that a thing is not a poison”

Postmortem Forensic Toxicology N N N Suspected drug intoxication cases Homicides Arson fire deaths Motor vehicle fatalities Deaths due to natural causes Specimens N Blood – from the heart and from the femoral or jugular veins N Vitreous humor N Urine N Bile N Liver N Other – lung, spleen, stomach contents or brain

Postmortem Forensic Toxicology N Specimens N Blood – from the heart and from the femoral or jugular veins N Vitreous humor N Urine N Bile N Liver N Other – lung, spleen, stomach contents or brain

Postmortem Forensic Toxicology N Analytical Process N Separation N Identification N Confirmation N Quantitation

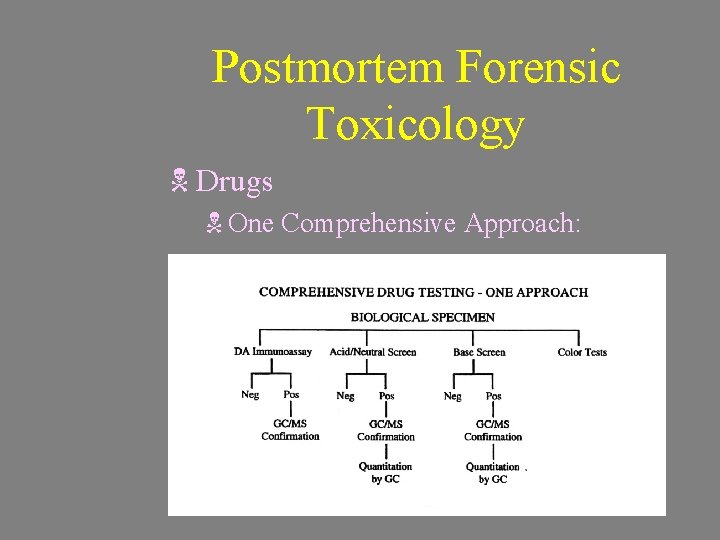
Postmortem Forensic Toxicology N Analytes N Volatiles (Carbon Monoxide, Cyanide, and Alcohols) N Drugs N Metals N Drugs N One Comprehensive Approach:

Postmortem Forensic Toxicology N Drugs N One Comprehensive Approach:

Postmortem Forensic Toxicology N Metals N Aluminum N Arsenic N Iron N Mercury N Lead N Thallium N Analysis N Colorimetric N Graphite Furnace Atomic Absorption Spectrometry N Inductively Coupled Plasma – Mass Spectrometry N Neutron Activation Spectrometry

Human Performance Toxicology N Human performance toxicology is also referred to as behavioral toxicology. N It is the study of human performance under the influence of drugs. N Ethanol and driving N History N Behavioral effect N Specimens N Types of alcohol N Ethanol (ethyl alcohol) N Methanol (methyl alcohol) N Isopropanol N Ethylene glycol

Ethanol Toxicology N Ethanol production N Fermentation of sugar or starch N Can only achieve 20% ethanol N Distillation N Distilled alcoholic beverages are usually 40 to 50% ethanol by volume (80 -100 proof)

Ethanol Pharmacokinetics N Absorption N Means of absorption N Dermal N Inhalation N IV N Oral N Gastrointestinal tract N Presence of food. N Distribution N Gastrointestinal tract N Portal vein N Liver N Heart N Lung N Heart N Body

Ethanol Pharmacokinetics N Elimination N 5 -10% in the urine N Saliva, expired air and sweat N Liver (enzymatic oxidation to acetaldehyde, acetic acid and carbon dioxide)

Ethanol Effects on the Body N Cardiovascular system N Central nervous system N Gastrointestinal tract N Kidney N Liver

N Breath Ethanol Testing Theory N Henry’s law N Ethanol in breath Vs ethanol in blood N 2100 to 1 ratio N 2300 to 1 ratio N Types of analyzers N Chemical N Reaction of ethanol with potassium dichromate/sulfuric acid solution N Colored solution that results is measured spectrophotometrically N IR spectrophotometry N Electrochemical oxidation - fuel cell

Breath Ethanol Testing N IR Spectrophotometry N Based on absorbance of light by the ethanol molecule N Mainstay in evidential breath testing devices N Electrochemical Oxidation N Oxidation of ethanol to acetic acid N Also used in evidential breath testing

Blood Ethanol Testing N Chemical N Screening N Quantitative N Disadvantage - aldehydes and ketones will interfere with the test N Enzymatic N Conversion of NAD to NADH by ethanol (serum, urine and whole blood) N Measured spectrophotometrically at 340 nm N Same reaction with a blue dye (thiazoyl blue) (serum, urine, fresh blood and postmortem blood) N Measured with a fluorometer

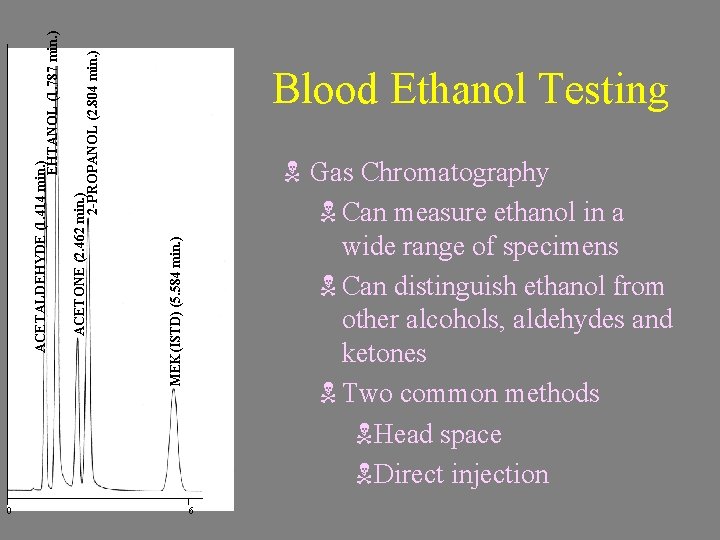
N Gas Chromatography N Can measure ethanol in a wide range of specimens N Can distinguish ethanol from other alcohols, aldehydes and ketones N Two common methods NHead space NDirect injection MEK(ISTD) (5. 584 min. ) ACETONE (2. 462 min. ) 2 -PROPANOL (2. 804 min. ) ACETALDEHYDE (1. 414 min. ) EHTANOL (1. 787 min. ) 0 Blood Ethanol Testing 6

Assessment of Ethanol Impairment N In a British study: N Detectable deterioration of drivers at between 30 – 50 mg/d. L N Obvious deterioration observed at between 60 – 100 mg/d. L N In another British study: N Pilots exhibited impairment at 40 mg/d. L N Blood alcohol concentration: N 10 -50 mg/d. L: Impairment detectable by special tests N 30 -120 mg/d. L: Beginning of sensorymotor impairment N 90 -250 mg/d. L: Sensory-motor incoordination; impaired balance N 180 -400 mg/d. L: Increased muscular incoordination; apathy; lethargy N 250 -400 mg/d. L: Impaired consciousness; sleep; stupor N 350 -500 mg/d. L: Complete unconsciousness; coma N 450 and greater mg/d. L: Death from respiratory arrest



Human Performance Toxicology N Drug Recognition Evaluation - 12 Step Process N N N Breath alcohol test Interview of the arresting officer. Preliminary examination of the suspect. Examination of the eyes. Divided attention psychophysical tests. Vital signs examination. Dark room examination. Examination of muscle tone. Examination for injection sites. Suspect’s statements and other observations. Opinion of the evaluator. Toxicological examination.
 Chapter 8 toxicology poisons and alcohol
Chapter 8 toxicology poisons and alcohol Toxicology types
Toxicology types Important people in forensics
Important people in forensics Forensic toxicology lab activity
Forensic toxicology lab activity Forensic toxicologist definition
Forensic toxicologist definition Forensic toxicology vocabulary
Forensic toxicology vocabulary Forensics toxicology definition
Forensics toxicology definition Forensic toxicology definition
Forensic toxicology definition Difference between primary secondary and tertiary alcohols
Difference between primary secondary and tertiary alcohols High boiling point alcohols
High boiling point alcohols Thomas mocker and thomas stewart
Thomas mocker and thomas stewart Forensic psychiatry vs forensic psychology
Forensic psychiatry vs forensic psychology Cardiac poisons
Cardiac poisons Erythism
Erythism Somniferous poisons
Somniferous poisons Clarke's analysis of drugs and poisons
Clarke's analysis of drugs and poisons Forensic psychology lecture
Forensic psychology lecture This is a collection of well-defined objects.
This is a collection of well-defined objects. 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Hormesis
Hormesis Examples of toxicology
Examples of toxicology Toxicology defination
Toxicology defination