Forensic Toxicology Definition The science of detecting and


















- Slides: 31

Forensic Toxicology • Definition: • The science of detecting and identifying the presence of drugs and poisons in body fluids, tissues, and organs.

Toxicology • Study of poisons or the detection of foreign substances in the body that can have a toxic effect such as: • Alcohol • Industrial chemicals • Poisonous gases • Illegal drugs • Drug overdoses

TOXICOLOGY • • TYPES: Environmental– air, water, soil Consumer– foods. Cosmetics, drugs Medical, clinical, forensic

Intoxicant vs. poison • An Intoxicant, such as alcohol or carbon monoxide, requires that you ingest a large amount to be lethal. • A true Poison, such as cyanide, requires only a very small amount

Drugs and Crime • Drug: a natural or synthetic substance designed to affect the subject psychologically or physiologically. • Controlled substance: drugs that are restricted by law • Controlled substances Act: enacted in 1970 lists illegal drugs, their cateogory and their penalty for possession, sale or use.

Toxicology plays a part in forensics at three levels 1. A criminalist may be asked to see if a person's behaviour has been influenced by a drug. 2. A forensic team may examine evidence to see whether a suspect has been manufacturing illicit compounds. 3. Forensic experts will look for evidence that at toxic substance has killed a person.

Role of the Toxicologist • Must identify one of thousands of drugs and poisons • Must find nanogram to microgram quantities dissipated throughout the entire body • Not always looking for exact chemicals, but metabolites of desired chemicals (ex. heroin morphine within seconds)

History • The father of toxicology was a guy named Mathieu Orfila. His work was mainly centered on arsenic; the poison of choice in the early 1800’s. it was readily available in rat poison and was the favourite means of murder among the poor people.

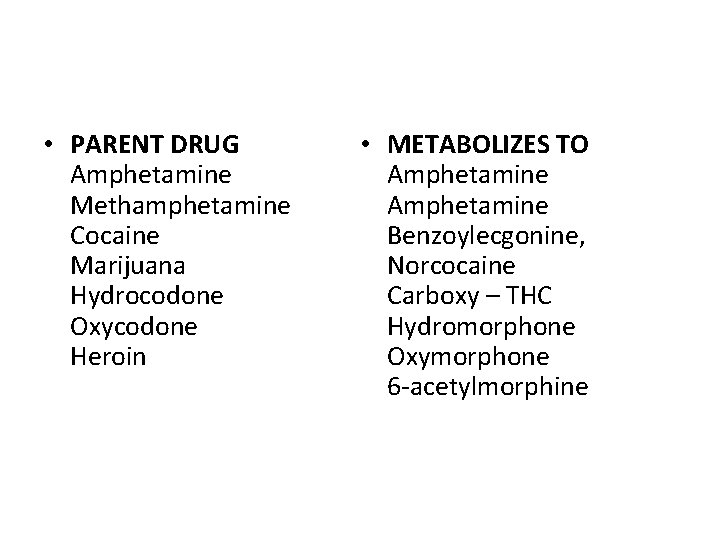
biotransformation When chemical enters the body, the body react by breaking it down in order to eliminate it. So, if you have injected something like heroin, the body will break it down into the morphine originally used to produce it. Hunting for heroin is futile but if you find morphine you have found signs of heroin. These products are called metabolites.

• PARENT DRUG Amphetamine Methamphetamine Cocaine Marijuana Hydrocodone Oxycodone Heroin • METABOLIZES TO Amphetamine Benzoylecgonine, Norcocaine Carboxy – THC Hydromorphone Oxymorphone 6 -acetylmorphine

What are some other common poisons? • Cyanide- one of the most lethal chemicals known. . used for executions. . causes a bright cherry red blood. • Strychnine-rat poisons. . causes so much pain that it is rarely used in suicide • Ethylene glycol- antifreeze. . a favourite (deadly) beverage among alcoholics when they cant get ethanol

Cont… • Heavy metals- arsenic mercury and lead • Insulin-lifesaving for diabetic but deadly overdoses • Corrosive chemicals-strong alkalis (Na. OH) or acids ( HCL, H 2 SO 4). . Burn the mouth, esophagus, and stomach.

Best tissues to sample for poisons • Blood- most useful tool will show chemicals and its metabolites. Blood levels show what was going on in the body at the time of death. • Urine- easy to obtain. High concentrations. Kidneys are along the elimination route • Stomach contents- digestion stops at the moment of death.

cont…. • Liver- the toxin sponge of your body can reflects levels of toxins that even the blood may not reveal • Vitreous humor- eyeball fluid very slow to decay so will retain toxins even longer then most other organs • Hair- chemicals take above five days to show up in the core of hair shafts.

What are the clues? • Ingested toxins show up in the stomach, intestines, or liver. • Inhaled gases are concentrated in the lungs. • Toxins that are injected intramuscularly concentrate themselves around the injection site. • Drugs that are given intravenously are directly absorbed into the blood by passing the stomach and liver. Concentrations are found throughout the body , are low in the stomach and liver and high in the blood stream.

Let’s talk about alcohol…. . Do not try this…. .

Alcohol facts • The most commonly abused drug • Blood-alcohol levels are directly proportional to the degree of intoxication and are expressed in grams percent ( no. of grams of alcohol/100 ml blood) • Acts on the CNS favouring the brain • Blood carries alcohol all cells of your body but mostly to the watery areas of your body

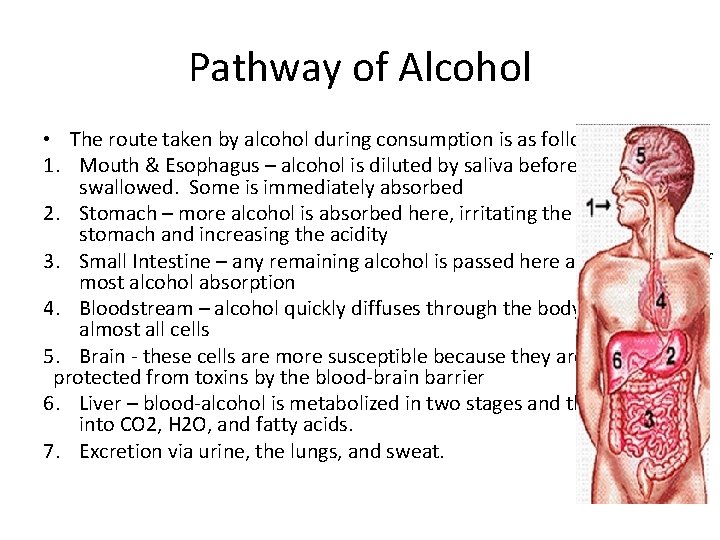
Pathway of Alcohol • The route taken by alcohol during consumption is as follows: 1. Mouth & Esophagus – alcohol is diluted by saliva before being swallowed. Some is immediately absorbed 2. Stomach – more alcohol is absorbed here, irritating the lining of the stomach and increasing the acidity 3. Small Intestine – any remaining alcohol is passed here and is the site of most alcohol absorption 4. Bloodstream – alcohol quickly diffuses through the body, affecting almost all cells 5. Brain - these cells are more susceptible because they are usually protected from toxins by the blood-brain barrier 6. Liver – blood-alcohol is metabolized in two stages and then respired into CO 2, H 2 O, and fatty acids. 7. Excretion via urine, the lungs, and sweat.

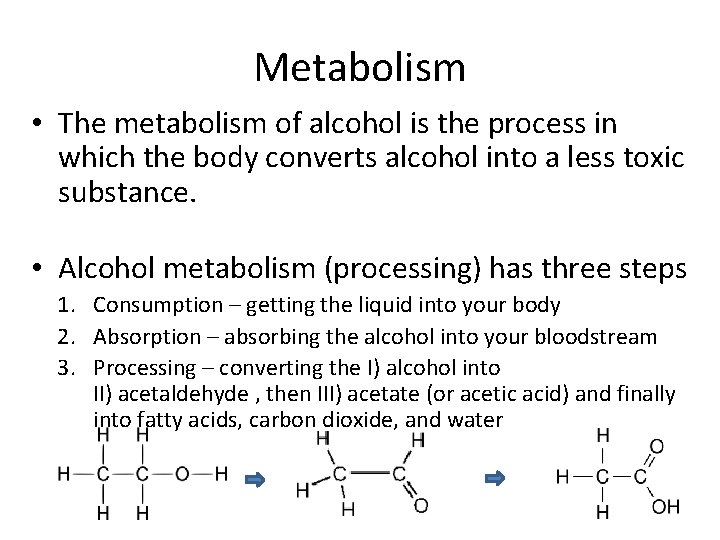
Metabolism • The metabolism of alcohol is the process in which the body converts alcohol into a less toxic substance. • Alcohol metabolism (processing) has three steps 1. Consumption – getting the liquid into your body 2. Absorption – absorbing the alcohol into your bloodstream 3. Processing – converting the I) alcohol into II) acetaldehyde , then III) acetate (or acetic acid) and finally into fatty acids, carbon dioxide, and water

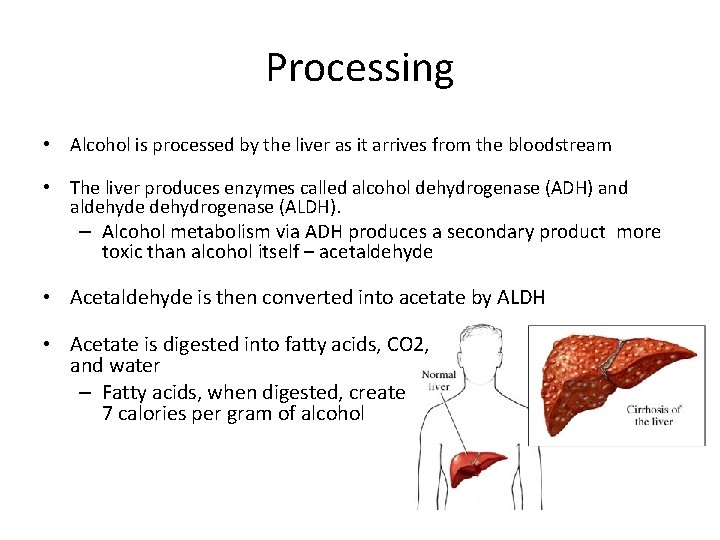
Processing • Alcohol is processed by the liver as it arrives from the bloodstream • The liver produces enzymes called alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH). – Alcohol metabolism via ADH produces a secondary product more toxic than alcohol itself – acetaldehyde • Acetaldehyde is then converted into acetate by ALDH • Acetate is digested into fatty acids, CO 2, and water – Fatty acids, when digested, create 7 calories per gram of alcohol

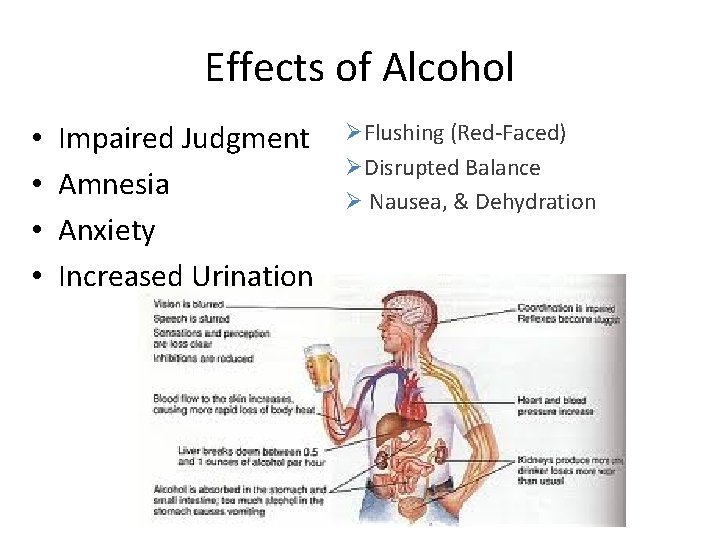
Effects of Alcohol • • Impaired Judgment Amnesia Anxiety Increased Urination ØFlushing (Red-Faced) ØDisrupted Balance Ø Nausea, & Dehydration

Toxicology Procedures • Screening– quick test to narrow down possibilities – color tests, TLC, GC, immunoassay • Confirmation– determines exact identity – GC/Mass Spectrometry Note: TLC—thin layer chromatography

Color Tests • Marquis Test: – Turns purple in the presence of Heroin, morphine, opium – Turns orange-brown in presence of Amphetamines • Scott Test: Three solutions – Blue then pink then back to blue in the presence of Cocaine • Duquenois-Levine: – Test for marijuana –turns purple

More Analytical Tests • Microcrystalline Tests: Identifies drug by using chemicals that reacts to produce characteristic crystals • Chromatography: TLC, HPLC and gas – separate drugs/tentative ID • Mass Spectrometry: chemical “fingerprint” no two drugs fragment the same

Microcrystalline Testing • In this technique, a small amount of an isolated sample is combined with a specific chemical reagent. If a certain drug or poison is present, a chemical reaction occurs, producing a crystalline precipitate. The crystalline structure and colour vary according to the drug or poison being tested. After the precipitate has formed, it may be analyzed under a microscope to confirm its identity.

Immunoassay Testing • Immunoassay testing identifies and measures the level of a drug or poison in an isolated sample. It uses the chemical reactions of antibodies to their specific antigens. Immunoassay testing is common because it is able to detect and accurately determine the concentration of the drug or poison in an isolated sample.

Cont… • Antibodies for drugs and poisons are produced in animal test subjects by combining the drug or poison with a protein to produce a drug-protein complex. Then, this is injected into the animal where it is perceived by the animal’s immune system as an antigen. Consequently, the animal� immune systems produces specific antibodies against this complex. Then, these antibodies are collected from the blood of the animal and used in immunoassay testing.

Gas Chromatography • Gas chromatography separates an isolated drug or poison sample into its distinctive component chemical parts. Gas chromatography is common because it is accurate and fast. Each drug or poison creates a predictable and distinctive peak or series of peaks that emerge at predictable times.

Cont… • Therefore, each can be identified easily in a chromatogram. The quantity of the individual drug or poison corresponds to the height of the peak(s) on the chromatogram. Thus, the higher the peak(s), the higher the concentration of drug or poison within the individual sample.

Mass Spectrometry • Every drug or poison produces a distinct fragmentation pattern according to its unique individual chemical structure. No two patterns are exactly alike. Because of this, the results of mass spectrometry are highly accurate. Mass spectrometry is an excellent way to confirm the presence of a particular drug or poison.

Thin Layer Chromatography • An adaptation of thin-layer chromatography for the isolation and identification of a number of the commercially available barbiturates and nonbarbiturate hypnotics is described. The method is rapid, inexpensive and simple. The hypnotics are extracted directly from urine, blood or tissue homogenate, without prior precipitation of proteins, into methylene chloride at p. H 5. 0. After evaporation of the extracting solvent, the drugs are taken up in a small volume of ethanol and spotted on thin-layer chromatoplates.
 Definition of forensic toxicology
Definition of forensic toxicology Forensic toxicologist definition
Forensic toxicologist definition Forensics toxicology definition
Forensics toxicology definition Leone lattes contribution to forensic science
Leone lattes contribution to forensic science Forensic toxicology lab activity
Forensic toxicology lab activity Forensic toxicology vocabulary
Forensic toxicology vocabulary Pathologist and anthropologist
Pathologist and anthropologist Who is this
Who is this What is forensic science definition
What is forensic science definition Deductive reasoning problem solving examples
Deductive reasoning problem solving examples Observation in forensic science
Observation in forensic science Forensic
Forensic Keratin forensic science definition
Keratin forensic science definition Deductive reasoning definition forensics
Deductive reasoning definition forensics Environmental toxicology definition
Environmental toxicology definition Toxicology definition
Toxicology definition Sniffer for detecting lost mobiles
Sniffer for detecting lost mobiles Detecting evolutionary forces in language change
Detecting evolutionary forces in language change Intrusion.win.ms14-068.ta
Intrusion.win.ms14-068.ta How do fraud symptoms help in detecting fraud
How do fraud symptoms help in detecting fraud Havex
Havex My favourite subject worksheet
My favourite subject worksheet Forensic science fundamentals and investigations chapter 6
Forensic science fundamentals and investigations chapter 6 A study of fibers and textiles chapter 4 review answer key
A study of fibers and textiles chapter 4 review answer key Forensic science chapter 1
Forensic science chapter 1 Hairs and fibers in forensic science
Hairs and fibers in forensic science Chapter 8 toxicology poisons and alcohol
Chapter 8 toxicology poisons and alcohol Chapter 8 toxicology test
Chapter 8 toxicology test Toxicology and applied pharmacology
Toxicology and applied pharmacology Annual review of pharmacology and toxicology
Annual review of pharmacology and toxicology Food safety and toxicology
Food safety and toxicology Drug identification and toxicology
Drug identification and toxicology