Forensic Toxicology Forensic Toxicology Definition The science of































- Slides: 38

Forensic Toxicology

Forensic Toxicology • Definition: • The science of detecting and identifying the presence of drugs and poisons in body fluids, tissues, and organs.

Controlled Substances Act • Federal Law established 5 schedules of classification of controlled substances based on – Drug’s potential for abuse – Potential to physical and psychological dependence – Medical Value • Note: Federal law also controls materials that are used in making drugs and those that are manufactured to resemble drugs

Drug Schedules • Schedule I: • Drugs with high potential for abuse and addiction, NO medical value Ex: Heroin, LSD, Ecstasy, Marijuana • Schedule II: • Drugs with high potential for abuse and addiction, have some medical value with restrictions Ex: PCP, Cocaine, Amphetamines, Most Opiates, Some Barbiturates

Drug Schedules • Schedule III: • Drugs with less potential for abuse and addiction, currently acceptable for medical use Ex: Some Barbiturates, Codeine, Steroids • Schedule IV: • Drugs with low potential for abuse and addiction, currently acceptable for medical use Ex: Tranquilizers like Valium, Xanax, Librium

Drug Schedules • Schedule V: • Drugs with low potential abuse, medical use, lowest potential dependency • Ex: Some Opiates with Non-Narcotic Ingredients

Role of the Toxicologist • Must identify one of thousands of drugs and poisons • Must find nanogram to microgram quantities dissipated throughout the entire body • Not always looking for exact chemicals, but metabolites of desired chemicals (ex. heroin morphine within seconds)

Toxicology Procedures • 10 m. L of blood in airtight container – Add anticoagulant – Add preservative • 2 consecutive urine samples – Some drugs take a while to show up in urine (1 -3 days) • Vitreous humor • Hair samples

Toxicology Procedures • Screening– quick test to narrow down possibilities – color tests, TLC, GC, immunoassay • Confirmation– determines exact identity – GC/Mass Spec Note: TLC—thin layer chromatography

Color Tests • Marquis Test: – Turns purple in the presence of Heroin, morphine, opium – Turns orange-brown in presence of Amphetamines • Scott Test: Three solutions – Blue then pink then back to blue in the presence of Cocaine • Duquenois-Levine: – Test for marijuana –turns purple

More Analytical Tests • Microcrystalline Tests: Identifies drug by using chemicals that reacts to produce characteristic crystals • Chromatography: TLC, HPLC and gas – separate drugs/tentative ID • Mass Spectrometry: chemical “fingerprint” no two drugs fragment the same

Why? • Think of all the people that you have “heard” do drugs. • US drug manufacturers produce enough barbiturates and tranquilizers each year to give every person in the US 40 pills • (that’s about 12 billion pills) • 18, 000 out of 44, 000 annual traffic deaths are alcohol related and send over 2 million people to the hospital

Toxicology of Alcohol • Alcohol is absorbed through the stomach and intestine • Once absorbed, alcohol is: – Oxidized- in liver by alcohol dehydrogenase—turned into acidic acid – Excreted- by breath, perspiration, and kidneys—turned into carbon dioxide and water

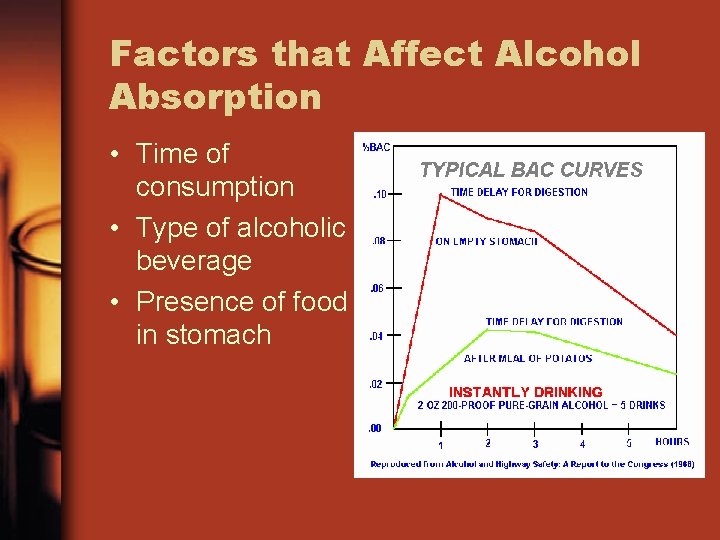
Factors that Affect Alcohol Absorption • Time of consumption • Type of alcoholic beverage • Presence of food in stomach

Toxicology of Alcohol • Alcohol intoxication depends on – Amount of alcohol consumed – Time of consumption – Body weight – Rate of alcohol absorption

Fate of Alcohol • Alcohol is absorbed into the bloodstream • Distributed through-out the body’s water • And finally eliminated by oxidation and excretion

Fate of Alcohol Con’t Note: A. Oxidation is the combination of oxygen and alcohol to produce new products by the liver B. Elimination is removing alcohol from the body in an unchanged state; normally excreted in breath and urine

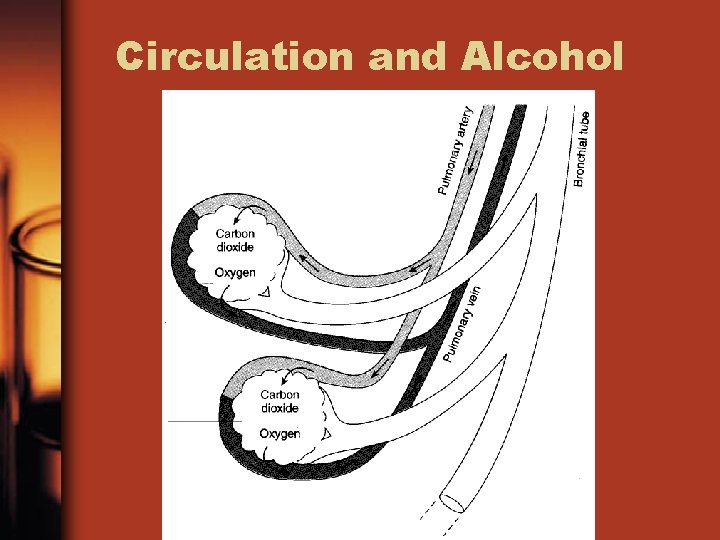
Alcohol in the Circulatory System • Measuring the quantity of alcohol in the blood system determines the degree to which someone is drunk • Two methods of making this measurement – Measurement of alcohol content in blood – Measurement of alcohol in breath

Circulation and Alcohol

Circulation and Alcohol

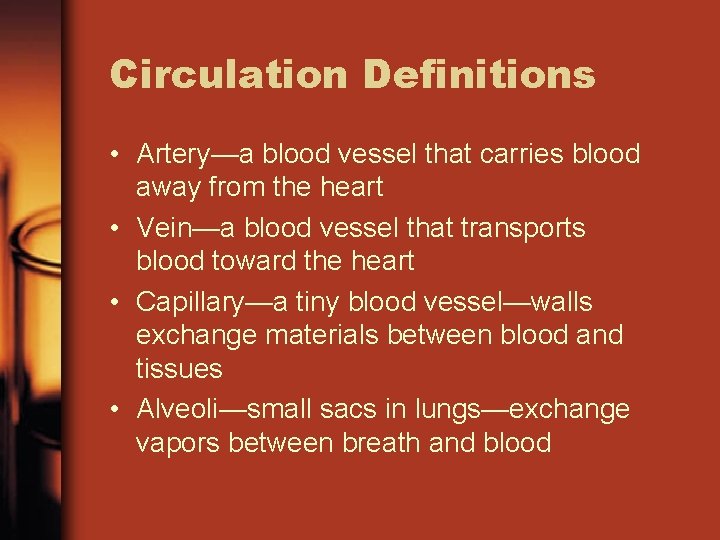
Circulation Definitions • Artery—a blood vessel that carries blood away from the heart • Vein—a blood vessel that transports blood toward the heart • Capillary—a tiny blood vessel—walls exchange materials between blood and tissues • Alveoli—small sacs in lungs—exchange vapors between breath and blood

Circulation Con’t • Note: If alcohol is present, it will be passed from the blood into the alveoli where it will be passed on to the mouth and nose during the act of breathing. • Evidence has shown that the ratio of alcohol to alveoli air is approx. 2100 to 1 —This is a basis for relating breath to blood-alcohol concentration.

Analysis of BAC • Breath Tests • Field Sobriety Tests • Blood Tests

Breath Tests • A breath test reflects the alcohol concentration in the pulmonary artery. • One instrument used for breath tests is called The Breathalyzer. • The Breathalyzer is a device for collecting and measuring the alcohol content of alveolar breath.

The Breathalyzer

The Breathalyzer Con’t • The Breathalyzer traps 1/40 of 2100 milliliters of alveolar breath. • Since the amount of alcohol in 2100 milliliters of breath approximates the amount of alcohol in 1 milliliter of blood—the Breathalyzer in essence measures the alcohol concentration present in 1/40 of a milliliter of blood.

Breathalyzer Con’t • Once the alveolar breath is trapped it is allowed to undergo a chemical reaction: • 2 K 2 Cr 2 O 7 + 3 C 2 H 5 OH + 8 H 2 SO 4 2 Cr 2(SO 4)3 + 2 K 2 SO 4 + 3 CH 3 COOH + 11 H 2 O Potassium dichromate Ethyl alcohol Sulfuric acid Chromium sulfate Potassium sulfate Acetic acid Dihydrogen oxide • The Breathalyzer indirectly determines the quantity of alcohol consumed by measuring the absorption of light by potassium chromate before and after its reaction with alcohol, using the principle of spectrophotometry

Other Breath Tests • Infrared breath-testing instrument • Fuel cell • Note: These instruments are used more recently because they don’t depend upon chemical reagents and are entirely automated.

Infrared-Breath Test • Uses the principle that infrared light is absorbed when shined on alcohol • Essentially, the infrared light passes through a chamber where it will interact with the alcohol and cause the light density to decrease. • The decrease in light intensity is proportional to the concentration of alcohol present in the captured breath

Fuel Cell—Breath Test • A fuel cell converts a fuel and an oxidant into an electrical current. • In this test, the breath alcohol is the fuel and atmospheric oxygen acts as the oxidant. • Alcohol is converted, generating a current that is proportional to the quantity of alcohol present in the breath.

Infrared and Fuel Cell Breath Tests • Infrared Breath Test uses infrared wavelengths to test for alcohol or other interferences in the breath • Fuel Cell Test converts fuel (alcohol) and oxygen into a measurable electric current

Field Sobriety Testing • Two reasons for the field sobriety test: 1. Used as a preliminary test to ascertain the degree of the suspect’s physical impairment 2. To see whether or not an evidential test is justified.

Field Sobriety Testing Methods • Field sobriety testing consists of a series of psychophysical tests and a preliminary breath test (typically done with a handheld fuel cell tester) • These tests are preliminary and nonevidential in nature—they only serve to establish probable cause requiring a more thorough breath or blood test.

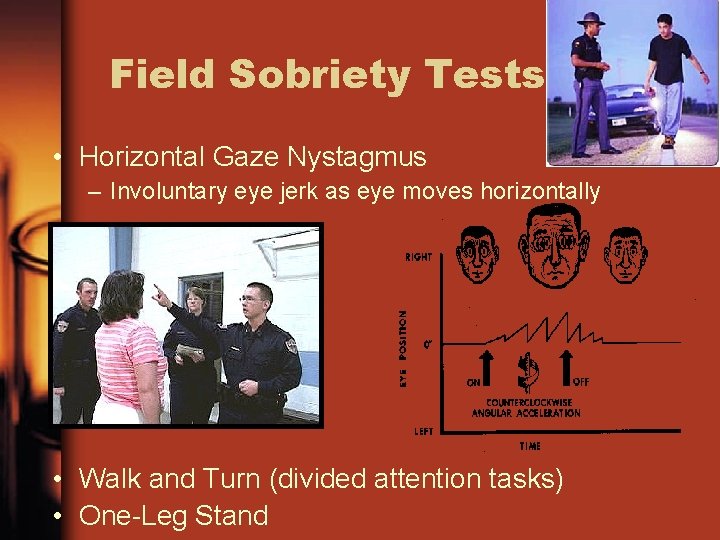
Field Sobriety Tests • Horizontal Gaze Nystagmus – Involuntary eye jerk as eye moves horizontally • Walk and Turn (divided attention tasks) • One-Leg Stand

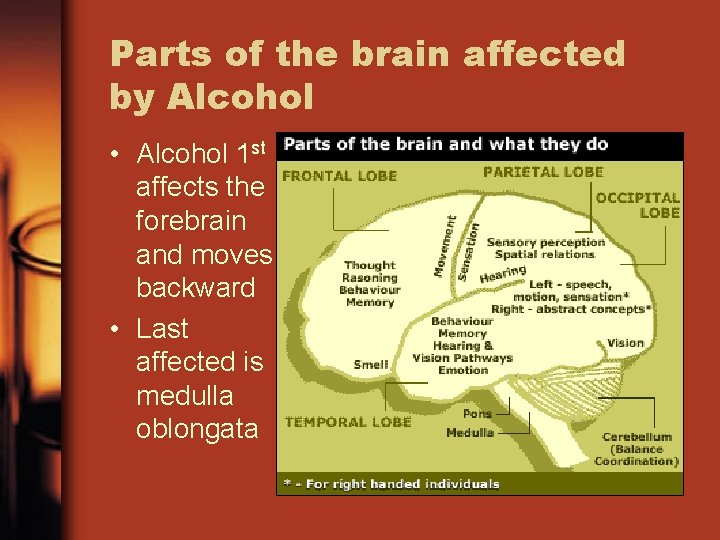
Parts of the brain affected by Alcohol • Alcohol 1 st affects the forebrain and moves backward • Last affected is medulla oblongata

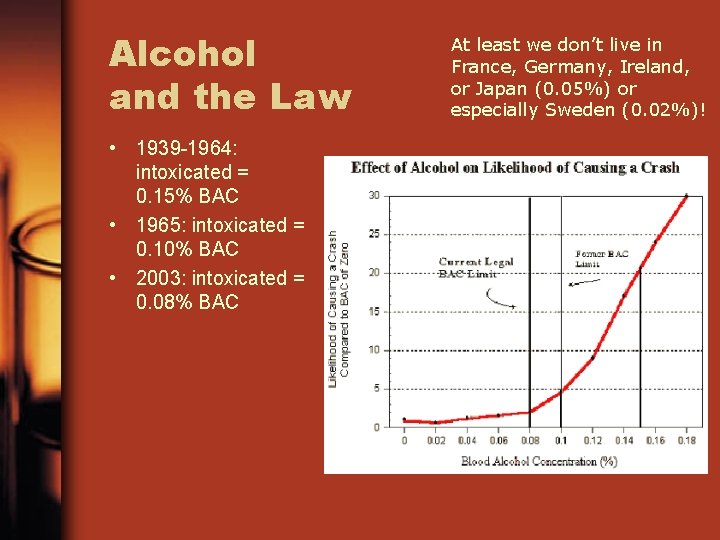
Alcohol and the Law • 1939 -1964: intoxicated = 0. 15% BAC • 1965: intoxicated = 0. 10% BAC • 2003: intoxicated = 0. 08% BAC At least we don’t live in France, Germany, Ireland, or Japan (0. 05%) or especially Sweden (0. 02%)!

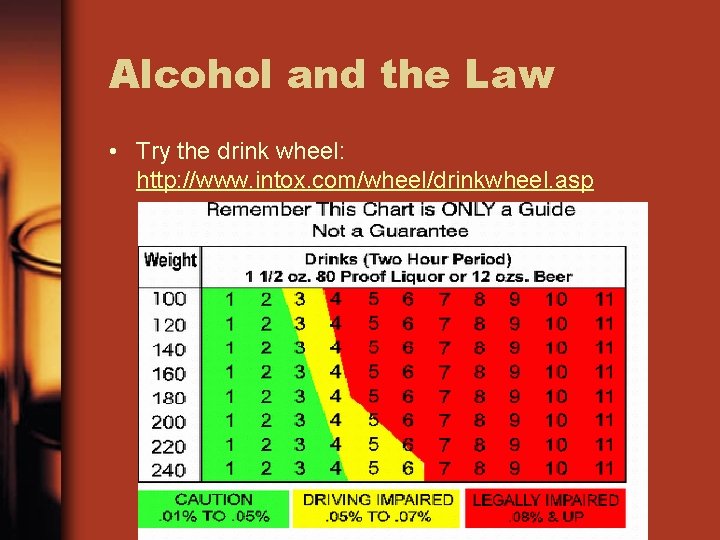
Alcohol and the Law • Try the drink wheel: http: //www. intox. com/wheel/drinkwheel. asp

The End
 A breath test reflects the alcohol concentration in the:
A breath test reflects the alcohol concentration in the: A breath test reflects the alcohol concentration in the:
A breath test reflects the alcohol concentration in the: Forensics toxicology definition
Forensics toxicology definition Forensic science timeline
Forensic science timeline Forensic toxicology lab activity
Forensic toxicology lab activity Forensic toxicology vocabulary
Forensic toxicology vocabulary What's your favourite subject
What's your favourite subject What is forensic science definition
What is forensic science definition Deductive reasoning definition forensic science
Deductive reasoning definition forensic science Observation definition forensics
Observation definition forensics Keratin forensic science definition
Keratin forensic science definition Forensic
Forensic Deductive reasoning definition forensic science
Deductive reasoning definition forensic science Forensic pathologist vs forensic anthropologist
Forensic pathologist vs forensic anthropologist Who is this
Who is this Define environmental toxicology
Define environmental toxicology Toxicology definition
Toxicology definition Hegar sign
Hegar sign Morphology definition forensics
Morphology definition forensics Mass spectrometry in forensic science
Mass spectrometry in forensic science Chain of custody forensics
Chain of custody forensics Ted bundy signature behaviour
Ted bundy signature behaviour Tire forensic examinations
Tire forensic examinations Serology forensic science
Serology forensic science Forensic science unit 1 review
Forensic science unit 1 review Forensic science foodborne outbreak investigation answers
Forensic science foodborne outbreak investigation answers Forensic science begins at the crime scene.
Forensic science begins at the crime scene. Forensic science arson activity
Forensic science arson activity The golden book of camping
The golden book of camping Hair and fiber evidence worksheet answers
Hair and fiber evidence worksheet answers Chapter 4 a study of fibers and textiles crossword
Chapter 4 a study of fibers and textiles crossword What is nibis
What is nibis Chapter 14 forensic anthropology
Chapter 14 forensic anthropology Forensic science branches
Forensic science branches Uv photography in forensic science
Uv photography in forensic science History of forensic science ppt
History of forensic science ppt Druggist fold definition
Druggist fold definition Department of forensic science dc
Department of forensic science dc Hans gross forensic science
Hans gross forensic science