Lecture 5 Major Genes Polygenes and QTLs Major
















- Slides: 32

Lecture 5: Major Genes, Polygenes, and QTLs

Major genes --- genes that have a significant effect on the phenotype Polygenes --- a general term of the genes of small effect that influence a trait QTL, quantitative trait locus --- a particular gene underlying the trait. Usually used when a gene underlying a trait is mapped to a particular chromosomal region Candidate gene --- a particular known gene that is of interest as being a potential candidate for contributing to the variation in a trait Mendelizing allele. The allele has a sufficiently large effect that its impact is obvious when looking at phenotype

Major Genes • Major morphological mutations of classical genetics that arose by spontaneous or induced mutation • Genes of large effect have been found selected lines – pygmy, obese, dwarf and hg alleles in mice – booroola F in sheep – halothane sensitivity in pigs • Major genes tend to be deleterious and are at very low frequencies in unselected populations, and contribute little to Var(A)

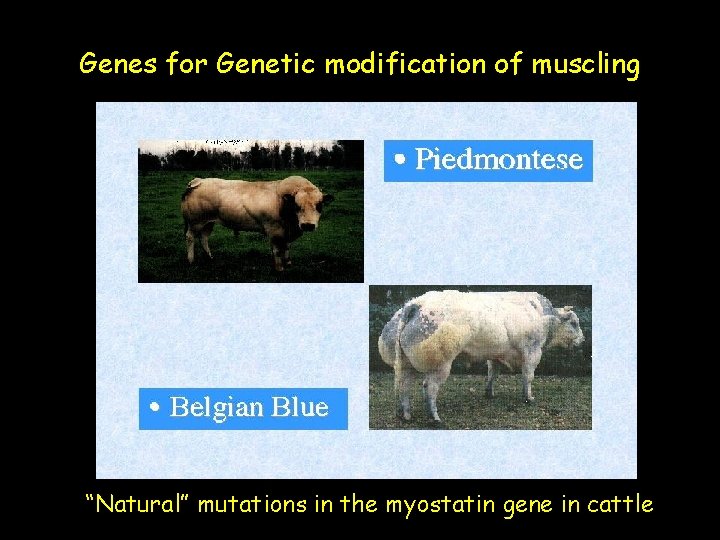
Genes for Genetic modification of muscling “Natural” mutations in the myostatin gene in cattle

“Natural” mutation in the callipyge - gene in sheep

“Booroola” gene in sheep increasing ovulation rate Merino Sheep

Major genes for mouse body size The mutations ob or db cause deficiencies in leptin production, or leptin receptor deficiencies

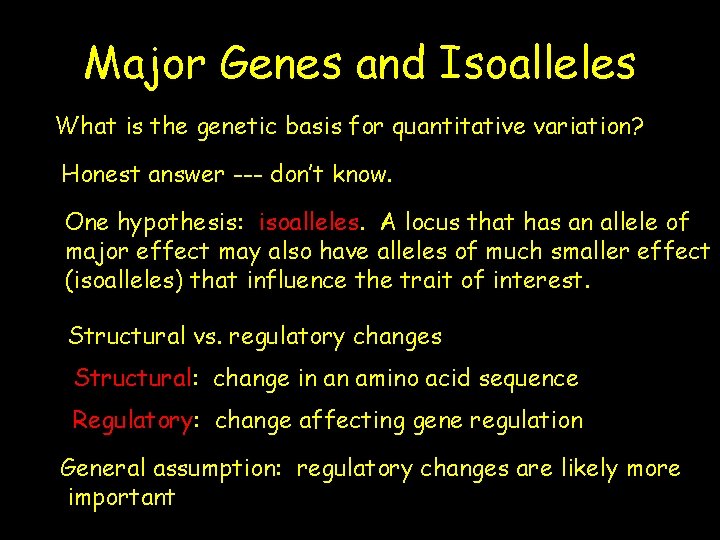
Major Genes and Isoalleles What is the genetic basis for quantitative variation? Honest answer --- don’t know. One hypothesis: isoalleles. A locus that has an allele of major effect may also have alleles of much smaller effect (isoalleles) that influence the trait of interest. Structural vs. regulatory changes Structural: change in an amino acid sequence Regulatory: change affecting gene regulation General assumption: regulatory changes are likely more important

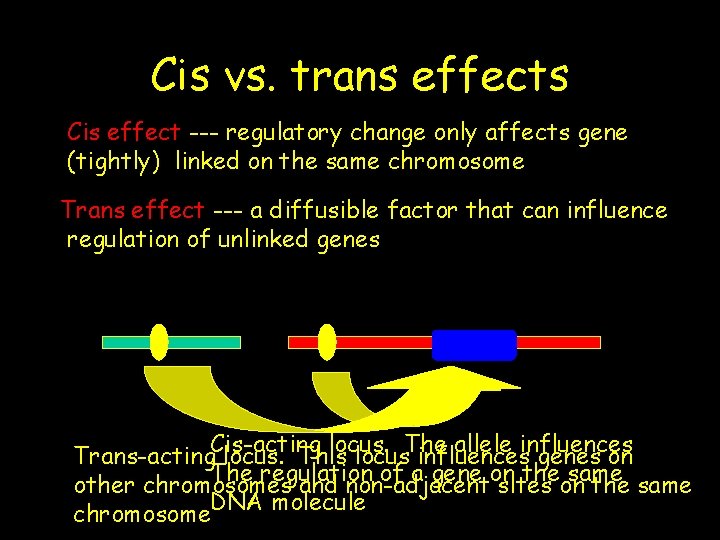
Cis vs. trans effects Cis effect --- regulatory change only affects gene (tightly) linked on the same chromosome Trans effect --- a diffusible factor that can influence regulation of unlinked genes Cis-acting locus. The allele influences Trans-acting locus. This locus influences genes on The regulation of a gene on the same other chromosomes and non-adjacent sites on the same DNA molecule chromosome

CIS-modifiers MASTER modifiers Genomic location of genes on array TRANS-modifiers Genomic location of m. RNA level modifiers

Polygenic Mutation For “normal” genes (i. e. , those with large effects) simply giving a mutation rate is sufficient (e. g. the rate at which an dwarfing allele appears) For alleles contributing to quantitative variation, we must account for both the rate at which mutants appear as well as the phenotypic effect of each Mutational variance, Vm or s 2 m - the amount of new additive genetic variance introduced by mutation each generation Typically Vm is on the order of 10 -3 VE

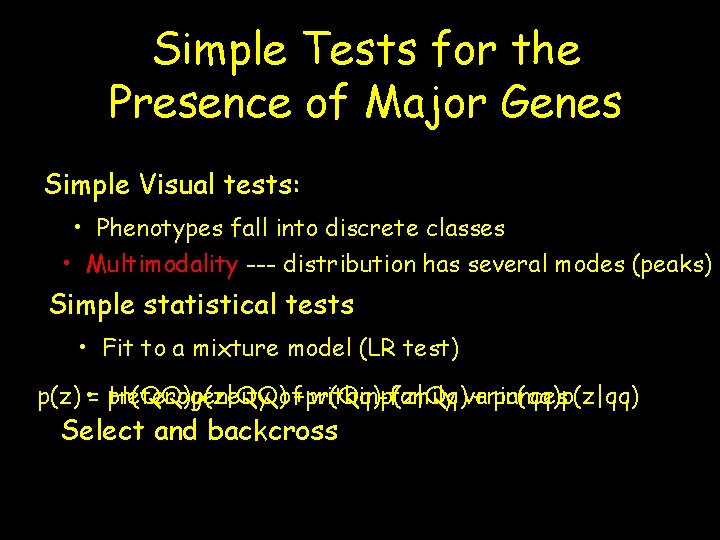
Simple Tests for the Presence of Major Genes Simple Visual tests: • Phenotypes fall into discrete classes • Multimodality --- distribution has several modes (peaks) Simple statistical tests • Fit to a mixture model (LR test) p(z) • = pr(QQ)p(z|QQ) Heterogeneity of+pr(Qq)p(z|Qq) within-family variances + pr(qq)p(z|qq) Select and backcross

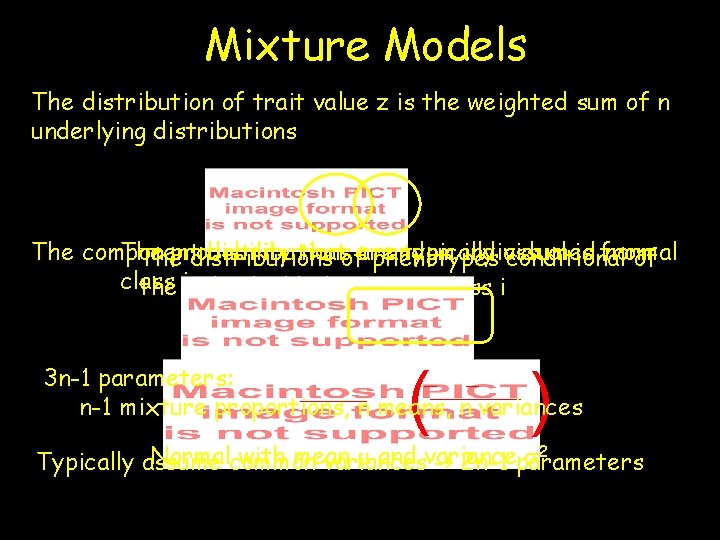
Mixture Models The distribution of trait value z is the weighted sum of n underlying distributions The probability thatofaare random individual is from The component distributions typically assumed normal The distributions phenotypes conditional of class the iindividual belonging to class i ( ) 3 n-1 parameters: n-1 mixture proportions, n means, n variances Normalcommon with mean m and variance s 2 Typically assume variances -> 2 n-1 parameters

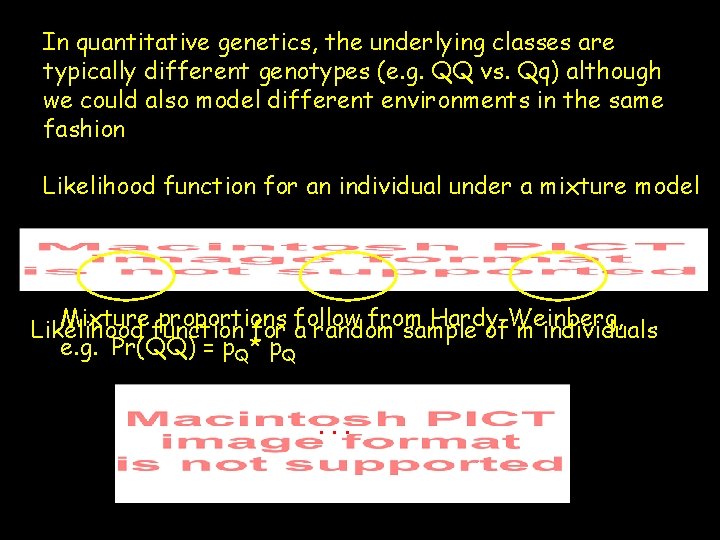
In quantitative genetics, the underlying classes are typically different genotypes (e. g. QQ vs. Qq) although we could also model different environments in the same fashion Likelihood function for an individual under a mixture model Mixture proportions follow from Hardy-Weinberg, Likelihood function for a random sample of m individuals e. g. Pr(QQ) = p. Q* p. Q. . .

Likelihood Ratio test for Mixtures Null hypothesis: A single normal distribution is adequate to fit the data. The maximum of the likelihood function under the null hypothesis is … The LR test for a significantly better fit under a mixture is given by 2 ln (max { likelihood under mixture}/max l 0 ) The LR follows a chi-square distribution with n-2 df, where n-1 = number of fitted parameters for the mixture

Complex Segregation Analysis A significant fit to a mixture only suggests the possibility of a major gene. A much more formal demonstration of a major gene is given by the likelihood-based method of Complex Segregation Analysis (CSA) Testing the fit of a mixture model requires a sample of random individuals from the population. CSA requires a pedigree of individuals. CSA uses likelihood to formally test for the transmission of A major gene in the pedigree

Building the likelihood for CSA Start with a mixture model Difference is that the mixing proportions are not the same for each individual, but rather are a function of its parental (presumed) genotypes Mean ofhaving genotype go Major-locus genotypes of parents Transmission Probability ofj Qq=2, QQ=1 an offspring genotype Phenotypic variance conditioned on major-locus genotype Example: code qq=3, Phenotypic value of individual in family i Likelihood i genotypes, Sum over for all family possible indexed goisgiven the parental genotypes are gf, gmby. g o =1, 2, 3 Sum over all possible parental genotypes Conditional family likelihood

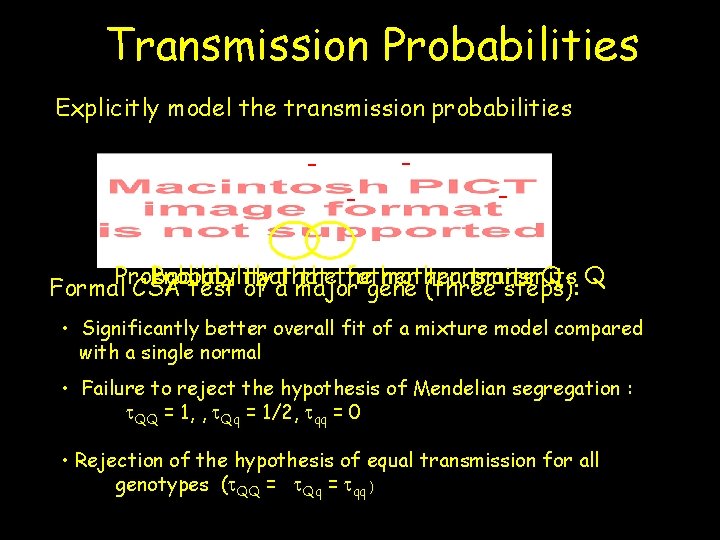
Transmission Probabilities Explicitly model the transmission probabilities - - - Probability that thethe father mother transmits Q Q Formal. Probability CSA test of athat major gene (three steps): • Significantly better overall fit of a mixture model compared with a single normal • Failure to reject the hypothesis of Mendelian segregation : t. QQ = 1, , t. Qq = 1/2, tqq = 0 • Rejection of the hypothesis of equal transmission for all genotypes (t. QQ = t. Qq = tqq )

CSA Modification: Common Family Effects Families can share a common environmental effect Expected value for go genotype, family i is mgo + ci Likelihood conditioned on common family effect ci Unconditional likelihood (average over all c --- assumed Normal with mean zero and variance sc 2 Likelihood function with no major gene, but family effects ( )

Maps and Mapping Functions The unit of genetic distance between two markers is the recombination frequency, c If the phase of a parent is AB/ab, then 1 -c is the frequency of “parental” gametes (e. g. , AB and ab), while c is the frequency of “nonparental” gametes (e. g. . Ab and a. B). A parental gamete results from an EVEN number of crossovers, e. g. , 0, 2, 4, etc. For a nonparental (also called a recombinant) gamete, need an ODD number of crossovers between A & b e. g. , 1, 3, 5, etc.

Hence, simply using the frequency of “recombinant” (i. e. nonparental) gametes UNDERESTIMATES the m number of crossovers, with E[m] > c In particular, c = Prob(odd number of crossovers) Mapping functions attempt to estimate the expected number of crossovers m from observed recombination frequencies c When considering two linked loci, the phenomena of interference must be taken into account The presence of a crossover in one interval typically decreases the likelihood of a nearby crossover

Suppose the order of the genes is A-B-C. If there is no interference (i. e. , crossovers occur independently of each other) then Probability(odd number of crossovers btw A and C) Odd number of crossovers A &of. B crossovers and even in Even inindependence A-B, btw odd number in B-C We need tonumber assume number & orderinterference tobetween multiply. Bthese two probabilities When is Cpresent, we can write this as Interference parameter d=0 --> complete No interference. Crossovers occur of 1 --> interference: The presence of each nearby other crossovers aindependently crossover eliminates

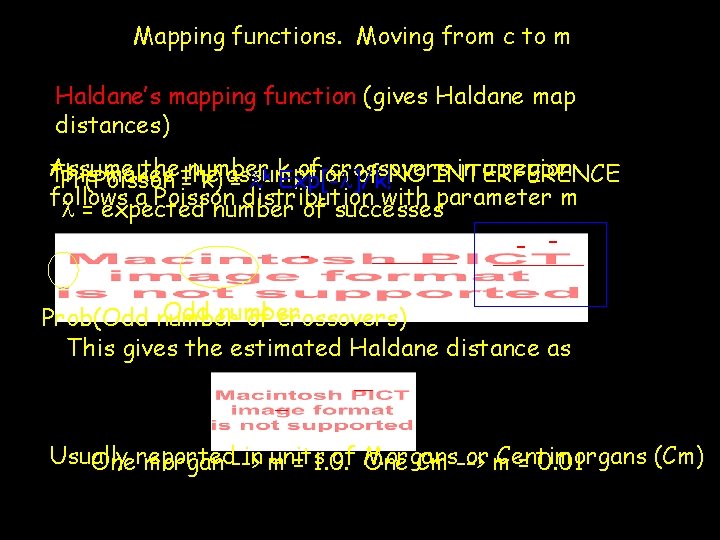
Mapping functions. Moving from c to m Haldane’s mapping function (gives Haldane map distances) Assume the of crossovers in a region This makes of NO INTERFERENCE Pr(Poisson =number k) assumption = lk k. Exp[-l]/k! follows a Poisson distribution with parameter m l = expected number of successes - - - Odd number Prob(Odd number of crossovers) This gives the estimated Haldane distance as Usually in m units of Morgans or m Centimorgans (Cm) Onereported morgan --> = 1. 0. One Cm --> = 0. 01

Linkage disequilibrium mapping Idea is to use a random sample of individuals from the population rather than a large pedigree. Ironically, in the right settings this approach has more power for fine mapping than pedigree analysis. Why? Key is the expected number of recombinants. in a pedigree, Prob(no recombinants) in n individuals is (1 -c)n LD mapping uses the historical recombinants in a sample. Prob(no recomb) = (1 -c)2 t, where t = Time back to most recent common ancestor

Expected number of recombinants in a sample of n sibs is cn Expected number of recombinants in a sample of n random individuals with a time t back to the MRCA (most recent common ancestor) is 2 cnt Hence, if t is large, many more expected recombinants in random sample and hence more power for very fine mapping (i. e. c < 0. 01) Because so many expected recombinants, only works with c very small

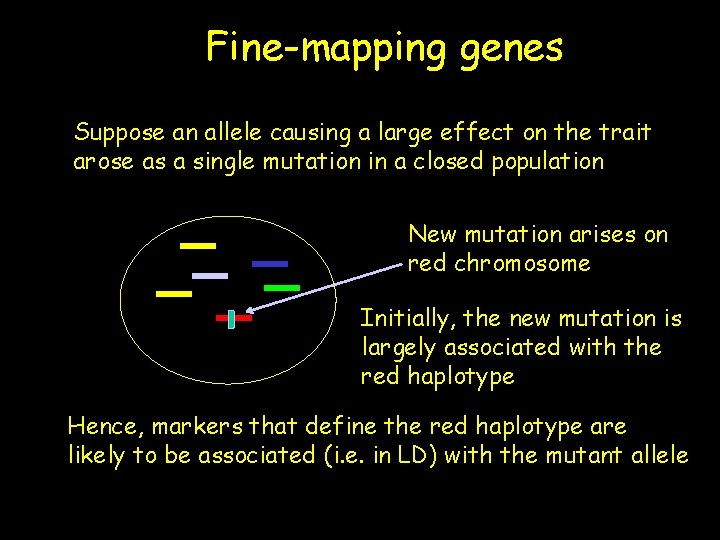
Fine-mapping genes Suppose an allele causing a large effect on the trait arose as a single mutation in a closed population New mutation arises on red chromosome Initially, the new mutation is largely associated with the red haplotype Hence, markers that define the red haplotype are likely to be associated (i. e. in LD) with the mutant allele

This linkage disequilibrium decays slowly with time if c is small Let p = Prob(mutation associated with original haplotype) p =(1 -c)t Thus if we can estimate p and t, we can solve for c, c = 1 - p 1/t

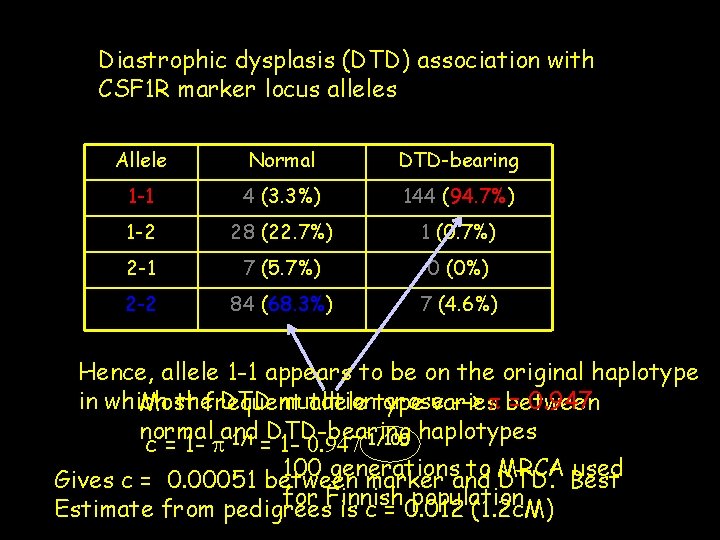
Diastrophic dysplasis (DTD) association with CSF 1 R marker locus alleles Allele Normal DTD-bearing 1 -1 4 (3. 3%) 144 (94. 7%) 1 -2 28 (22. 7%) 1 (0. 7%) 2 -1 7 (5. 7%) 0 (0%) 2 -2 84 (68. 3%) 7 (4. 6%) Hence, allele 1 -1 appears to be on the original haplotype in which thefrequent DTD mutation arose --> p between = 0. 947 Most allele type varies normal and DTD-bearing haplotypes 1/t 1/100 c = 1 - p = 1 - 0. 947 100 generations to MRCA used Gives c = 0. 00051 between marker and DTD. Best for Finnish population Estimate from pedigrees is c = 0. 012 (1. 2 c. M)

Candidate Loci and the TDT Often try to map genes by using case/control contrasts, also called association mapping. The frequencies of marker alleles are measured in both a case sample -- showing the trait (or extreme values) control sample -- not showing the trait The idea is that if the marker is in tight linkage, we might expect LD between it and the particular DNA site causing the trait variation. Problem with case-control approach: Population Stratification can given false positives.

When population being sampled actually consists of Example. The Gm marker was thought biological several distinct subpopulations we have(for lumped together, reasons)alleles to be may an excellent candidate gene marker provide information as tofor which group diabetes in the high-risk population of Pima indians an individual belongs. If there are other risk factors in the American Initially a verybtw strong aingroup, this can Southwest. create a false association marker and association was observed: trait Gm+ Total % with diabetes Present 293 8% Absent 4, 627 29% The association was+ re-examined in a population of Pima Problem: freq(Gm ) in Caucasians (lower-risk diabetes that were 7/8 th (or more) full heritage: Population) is 67%, Gm+ rare in full-blooded Pima Gm+ Total % with diabetes Present 17 59% Absent 1, 764 60%

Transmission-disequilibrium test (TDT) The TDT accounts for population structure. It requires sets of relatives and compares the number of times a marker allele is transmitted (T) versus not-transmitted (NT) from a marker heterozygote parent to affected offspring. Under the hypothesis of no linkage, these values should be equal, resulting in a chi-square test for lack of fit:

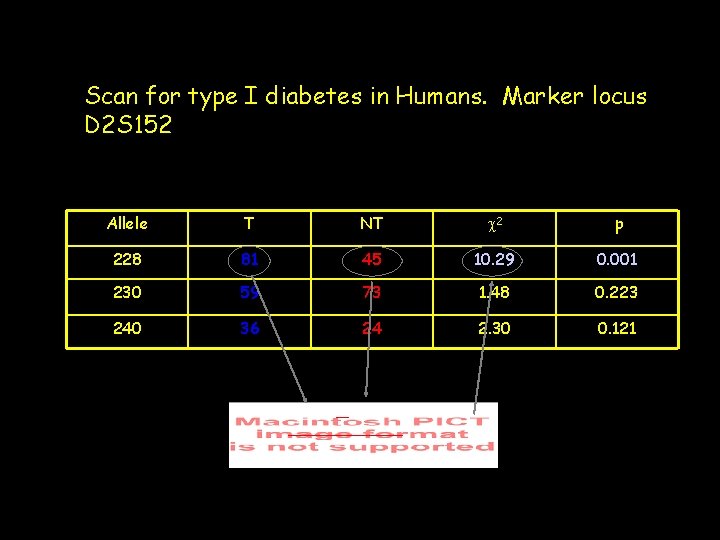
Scan for type I diabetes in Humans. Marker locus D 2 S 152 Allele T NT c 2 p 228 81 45 10. 29 0. 001 230 59 73 1. 48 0. 223 240 36 24 2. 30 0. 121
 Linked genes and unlinked genes
Linked genes and unlinked genes Linked genes and unlinked genes
Linked genes and unlinked genes Homeotic genes vs hox genes
Homeotic genes vs hox genes Etf qtls
Etf qtls Qtls workbook examples
Qtls workbook examples Institute for learning qtls
Institute for learning qtls 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Alleles are
Alleles are Stabilizing selection human birth weight
Stabilizing selection human birth weight The relationship between genes dna and chromosomes
The relationship between genes dna and chromosomes Learn genetics utah karyotype
Learn genetics utah karyotype Intermediate inheritance
Intermediate inheritance This section describes
This section describes Chapter 11 dna and genes
Chapter 11 dna and genes Evolution of populations section 16-1 genes and variation
Evolution of populations section 16-1 genes and variation Chapter 16 evolution of populations
Chapter 16 evolution of populations Dominant and recessive genes
Dominant and recessive genes Chromosomes genes and basic genetics foldable answer key
Chromosomes genes and basic genetics foldable answer key Dna, genes and chromosomes relationship
Dna, genes and chromosomes relationship Dna and genes chapter 11
Dna and genes chapter 11 Section 16-1 genes and variation
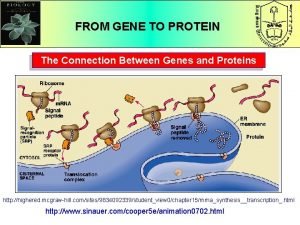
Section 16-1 genes and variation What is the connection between genes and proteins
What is the connection between genes and proteins Oncogenes and tumor suppressor genes
Oncogenes and tumor suppressor genes What is the relationship between dna chromosomes and genes
What is the relationship between dna chromosomes and genes Dna and genes chapter 11
Dna and genes chapter 11 Flocabulary genes and heredity answer key
Flocabulary genes and heredity answer key Linked genes
Linked genes Complementary genes example
Complementary genes example Punnett square blood type ab and o
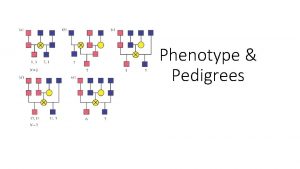
Punnett square blood type ab and o Phenotype pedigree chart
Phenotype pedigree chart Flujo de genes ejemplos
Flujo de genes ejemplos Cadena nucleotidica
Cadena nucleotidica Mendelian genetics
Mendelian genetics