Lecture 4 Symmetry and group theory Natural symmetry



















- Slides: 47

Lecture 4

Symmetry and group theory

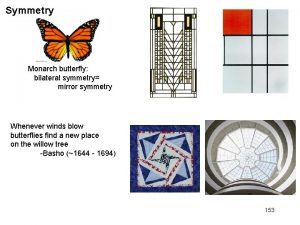
Natural symmetry in plants

Symmetry in animals

Symmetry in the human body

Symmetry in modern art M. C. Escher

Symmetry in arab architecture La Alhambra, Granada (Spain)

Symmetry in baroque art Gianlorenzo Bernini Saint Peter’s Church Rome

7 th grade art project Silver Star School Vernon, Canada

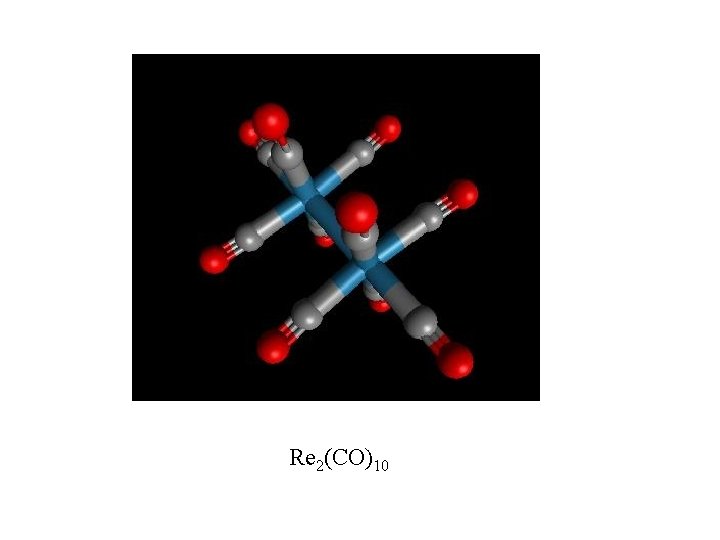
Re 2(CO)10

C 2 F 4 C 60

Symmetry in chemistry • Molecular structures • Wave functions • Description of orbitals and bonds • Reaction pathways • Optical activity • Spectral interpretation (electronic, IR, NMR). . .

Molecular structures A molecule is said to have symmetry if some parts of it may be interchanged by others without altering the identity or the orientation of the molecule

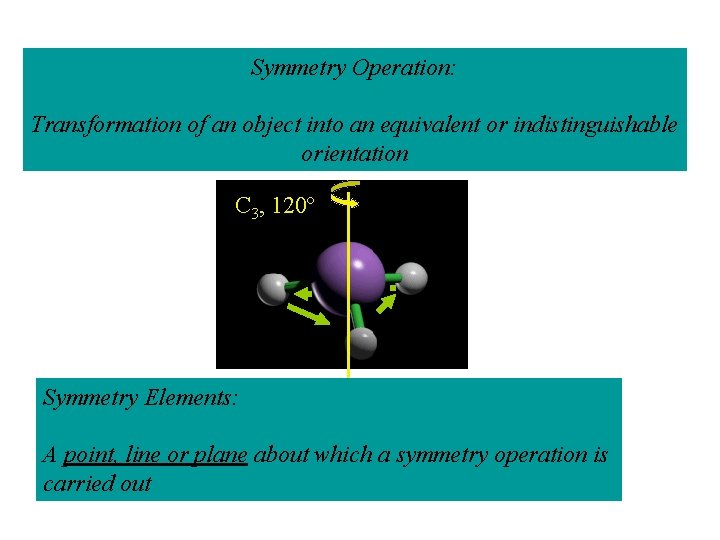
Symmetry Operation: Transformation of an object into an equivalent or indistinguishable orientation C 3, 120º Symmetry Elements: A point, line or plane about which a symmetry operation is carried out

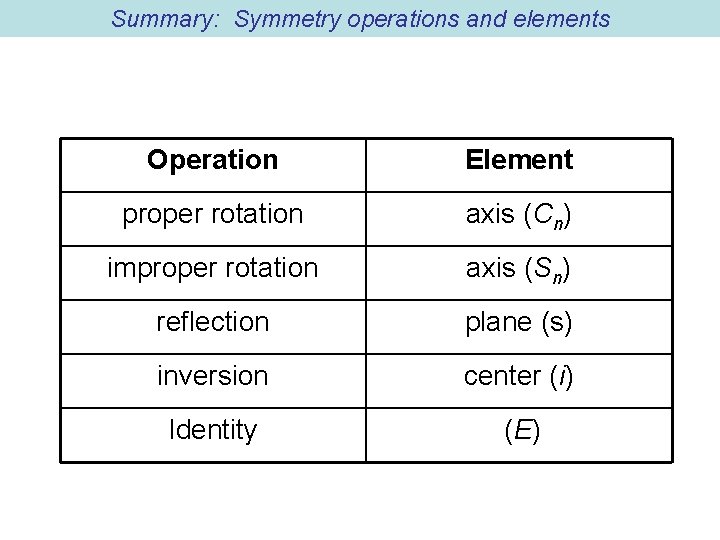
5 types of symmetry operations/elements Operation 1: Identity Operation, do nothing. Identity: this operation does nothing, symbol: E

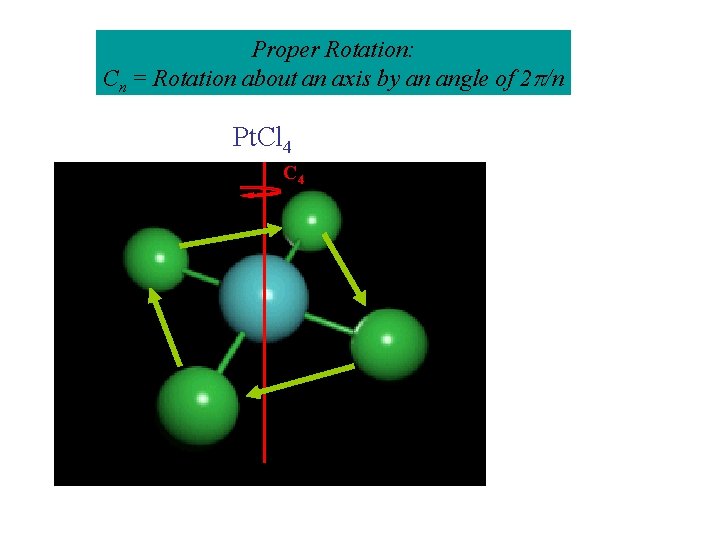
Operation 2: Cn, Proper Rotation: Rotation about an axis by an angle of 2 /n = 360/n C 2 H 2 O How about: C 3 NH 3 NFO 2?

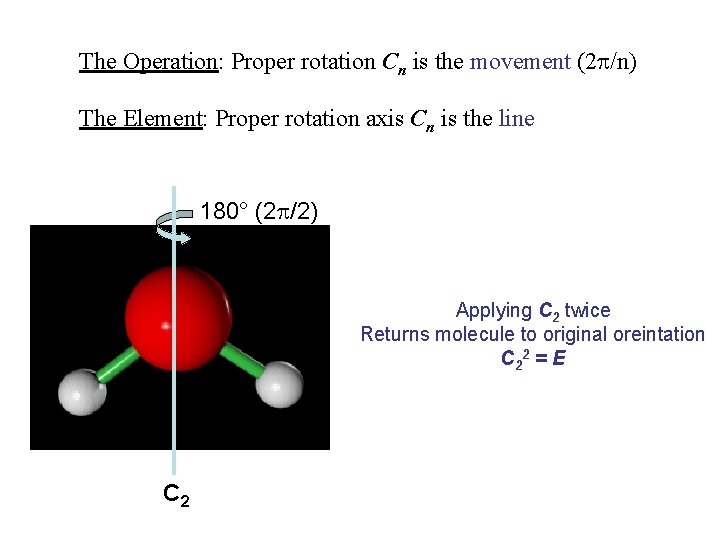
The Operation: Proper rotation Cn is the movement (2 /n) The Element: Proper rotation axis Cn is the line 180° (2 /2) Applying C 2 twice Returns molecule to original oreintation C 2 2 = E C 2

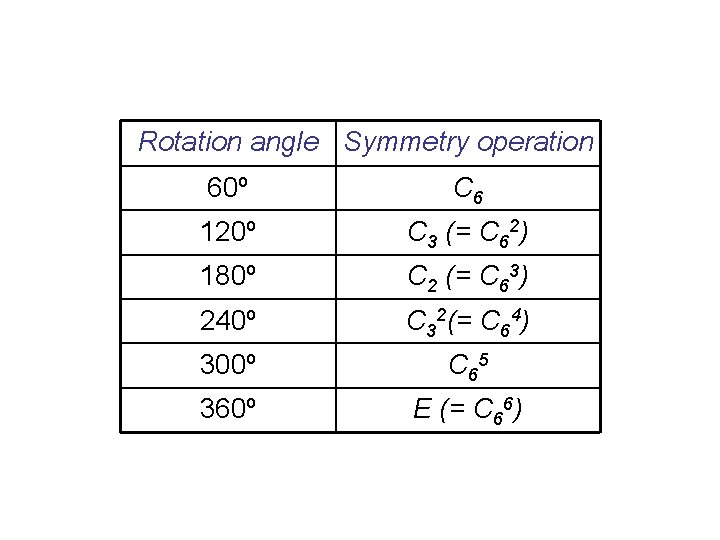
Rotation angle Symmetry operation 60º C 6 120º C 3 (= C 62) 180º C 2 (= C 63) 240º C 32(= C 64) 300º C 65 360º E (= C 66)

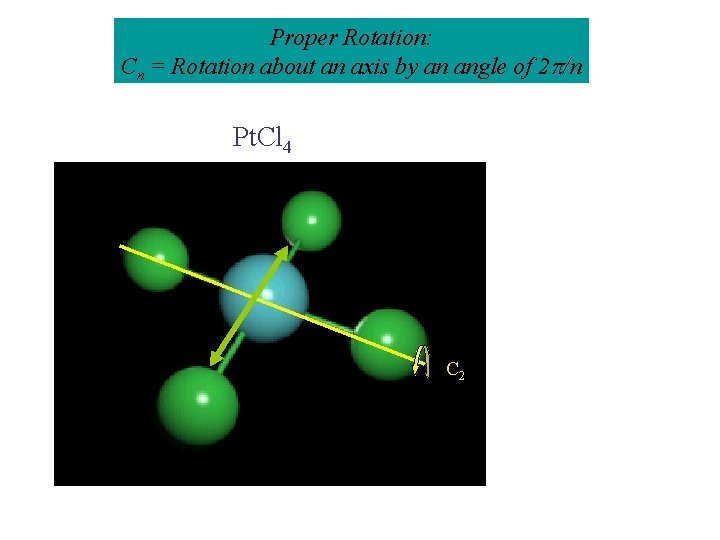
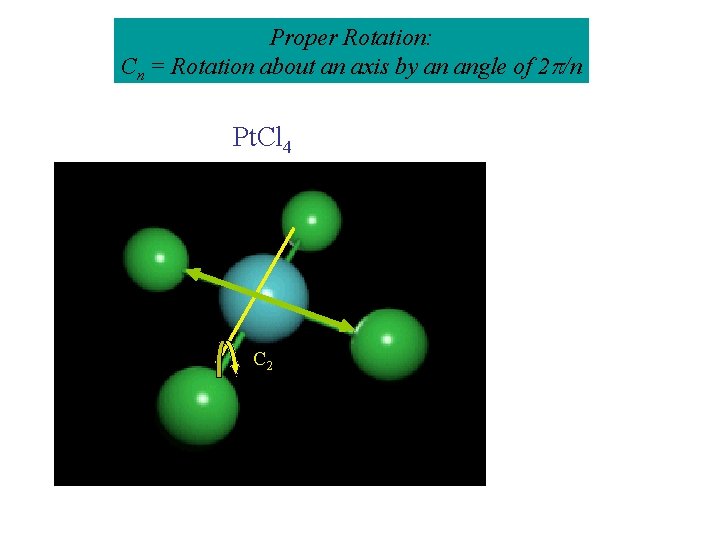
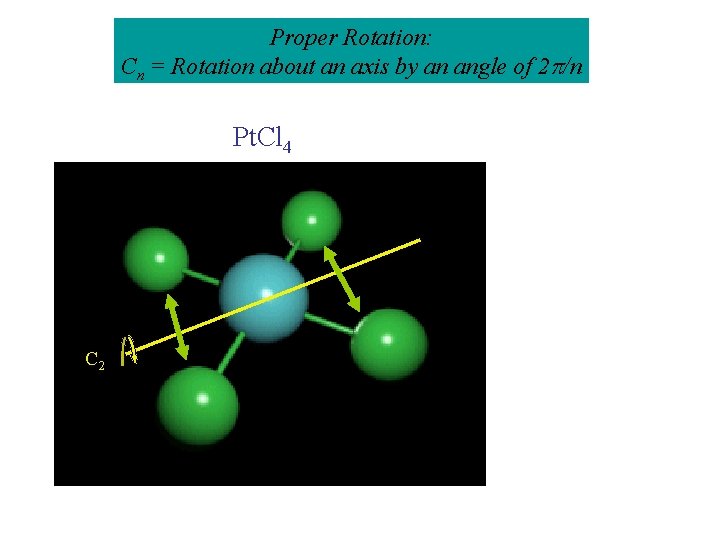
Proper Rotation: Cn = Rotation about an axis by an angle of 2 /n Pt. Cl 4 C 2

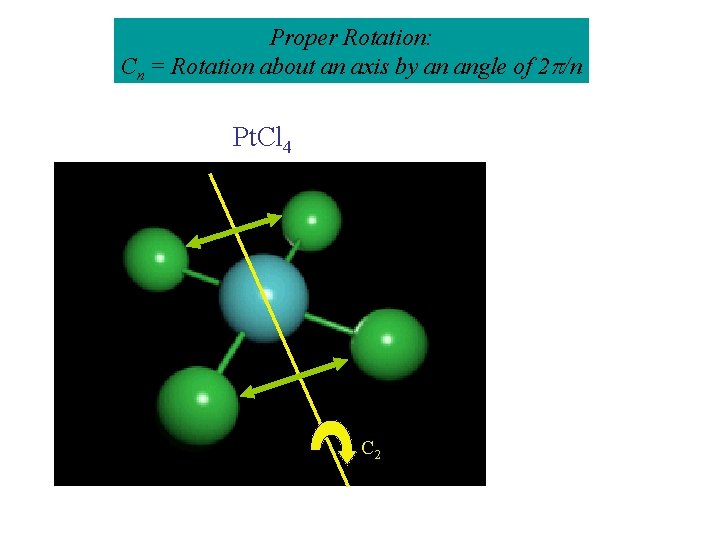
Proper Rotation: Cn = Rotation about an axis by an angle of 2 /n Pt. Cl 4 C 4

Proper Rotation: Cn = Rotation about an axis by an angle of 2 /n Pt. Cl 4 C 2

Proper Rotation: Cn = Rotation about an axis by an angle of 2 /n Pt. Cl 4 C 2

Proper Rotation: Cn = Rotation about an axis by an angle of 2 /n Pt. Cl 4 C 2

Proper Rotation: Cn = Rotation about an axis by an angle of 2 /n Pt. Cl 4 C 2

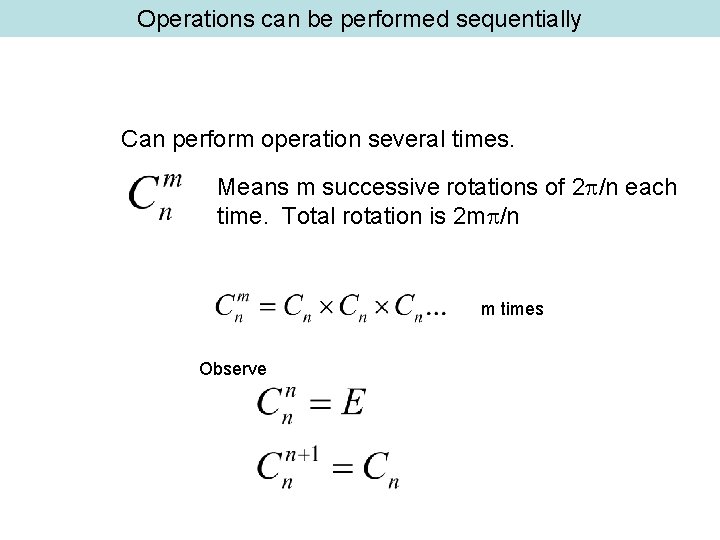
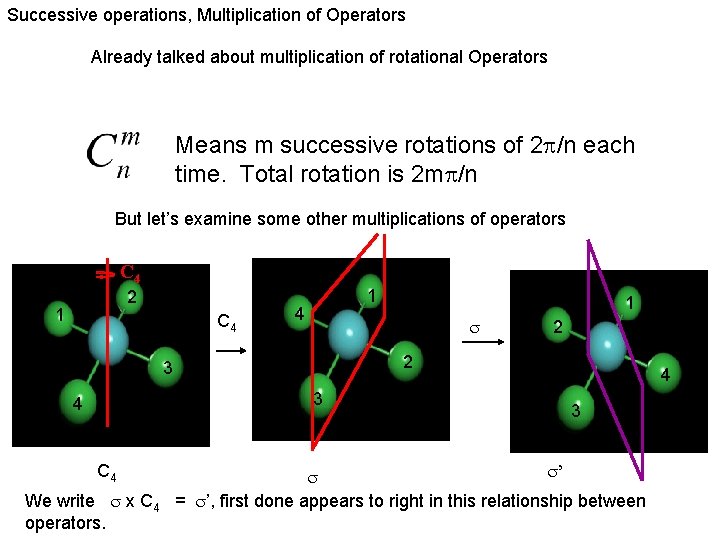
Operations can be performed sequentially Can perform operation several times. Means m successive rotations of 2 /n each time. Total rotation is 2 m /n m times Observe

C 3 axis Iron pentacarbonyl, Fe(CO)5 The highest order rotation axis is the principal axis and it is chosen as the z axis What other rotational axes do we have here?

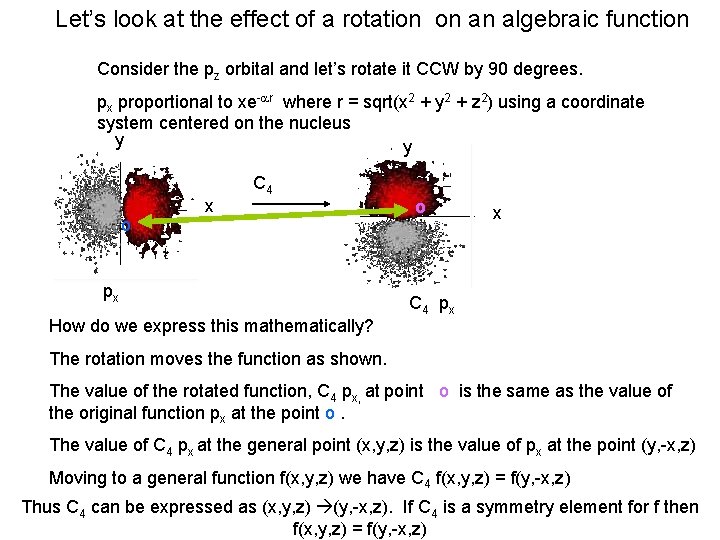
Let’s look at the effect of a rotation on an algebraic function Consider the pz orbital and let’s rotate it CCW by 90 degrees. px proportional to xe-ar where r = sqrt(x 2 + y 2 + z 2) using a coordinate system centered on the nucleus y y C 4 o x px o x C 4 px How do we express this mathematically? The rotation moves the function as shown. The value of the rotated function, C 4 px, at point o is the same as the value of the original function px at the point o. The value of C 4 px at the general point (x, y, z) is the value of px at the point (y, -x, z) Moving to a general function f(x, y, z) we have C 4 f(x, y, z) = f(y, -x, z) Thus C 4 can be expressed as (x, y, z) (y, -x, z). If C 4 is a symmetry element for f then f(x, y, z) = f(y, -x, z)

According to the pictures we see that C 4 px yields py. Let’s do it analytically using C 4 f(x, y, z) = f(y, -x, z) We start with px = xe-ar where r = sqrt(x 2 + y 2 + z 2) and make the required substitution to perform C 4 y y C 4 o px x o x C 4 px Thus C 4 px (x, y, z) = C 4 xe-ar = ye-ar = py And we can say that C 4 around the z axis as shown is not a symmetry element for px

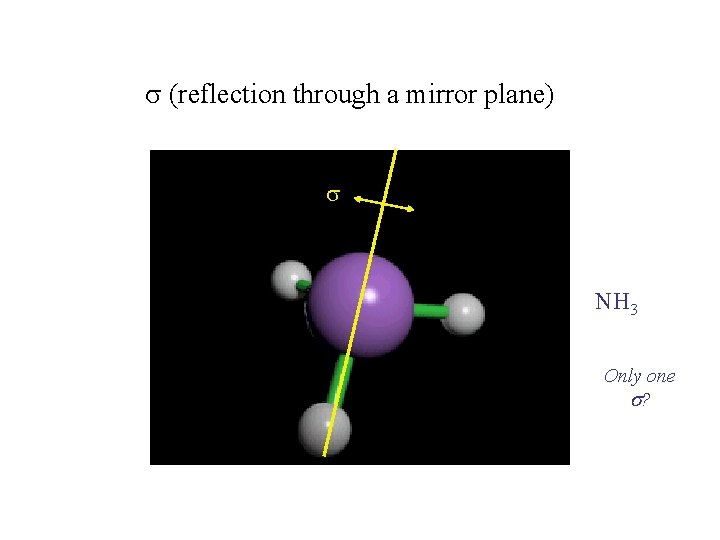
Operation 3: Reflection and reflection planes (mirrors) s s

(reflection through a mirror plane) NH 3 Only one s?

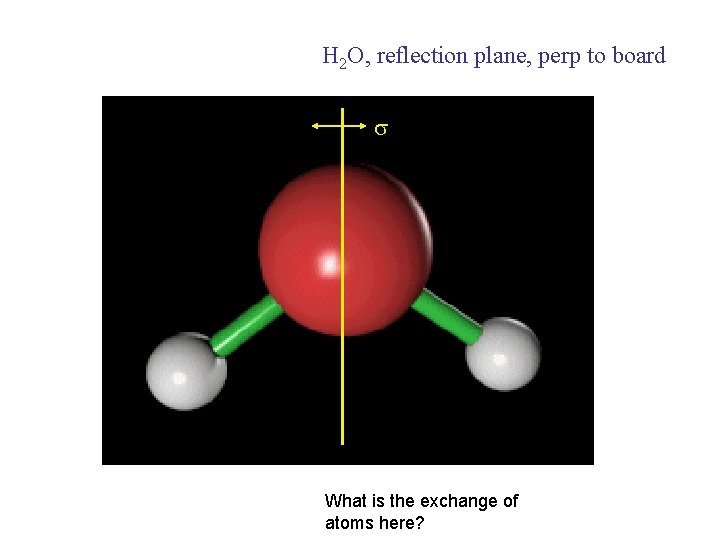
H 2 O, reflection plane, perp to board What is the exchange of atoms here?

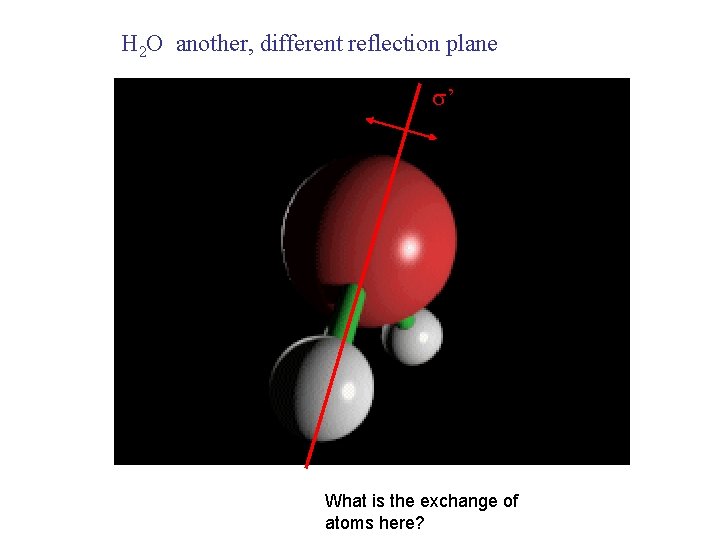
H 2 O another, different reflection plane ’ What is the exchange of atoms here?

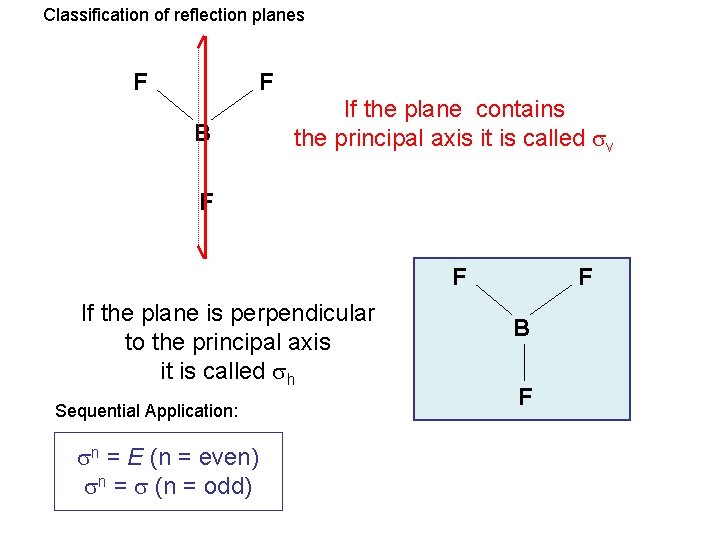
Classification of reflection planes F F B If the plane contains the principal axis it is called v F F If the plane is perpendicular to the principal axis it is called h Sequential Application: n = E (n = even) n = (n = odd) F B F

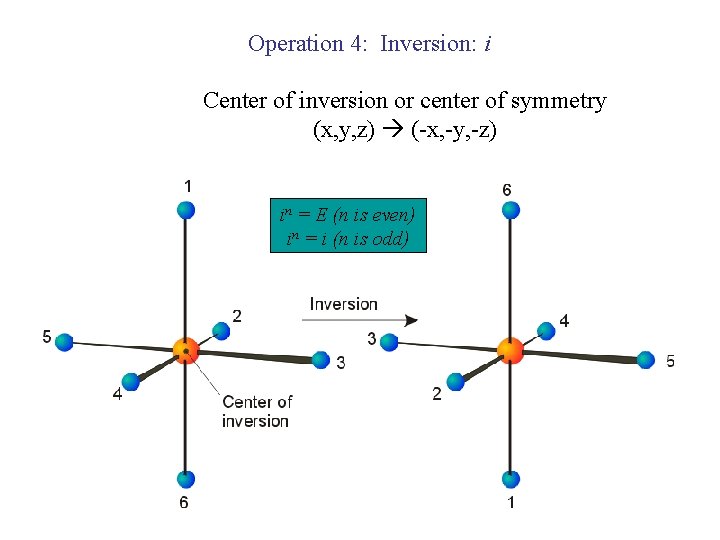
Operation 4: Inversion: i Center of inversion or center of symmetry (x, y, z) (-x, -y, -z) in = E (n is even) in = i (n is odd)

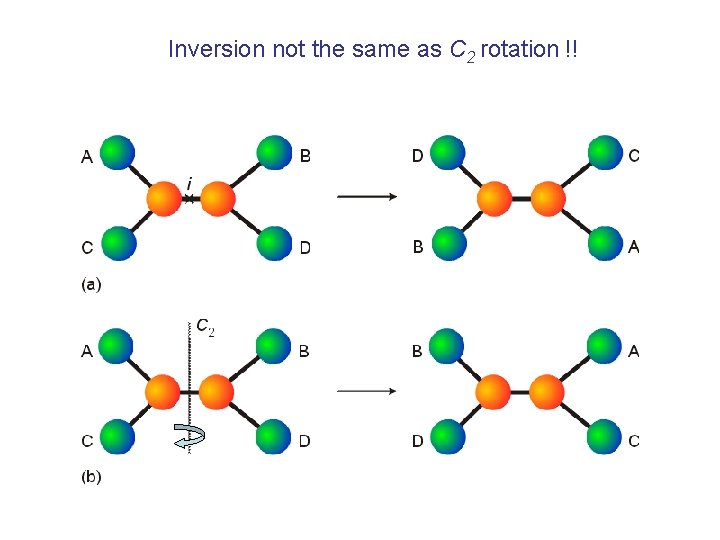
Inversion not the same as C 2 rotation !!


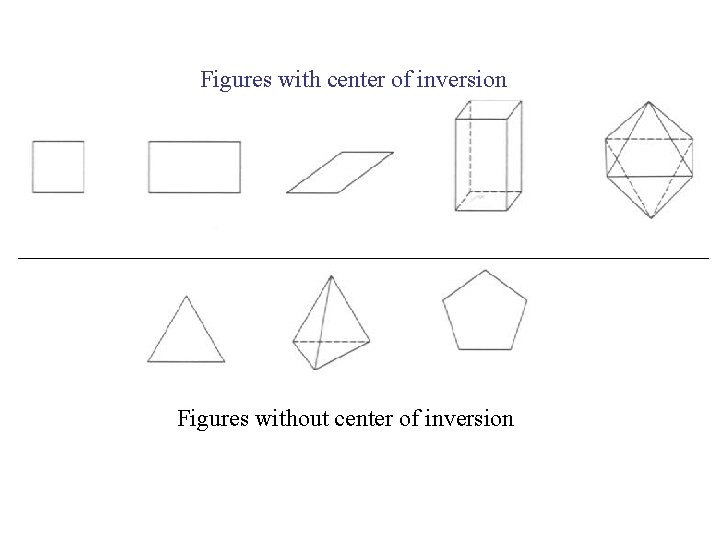
Figures with center of inversion Figures without center of inversion

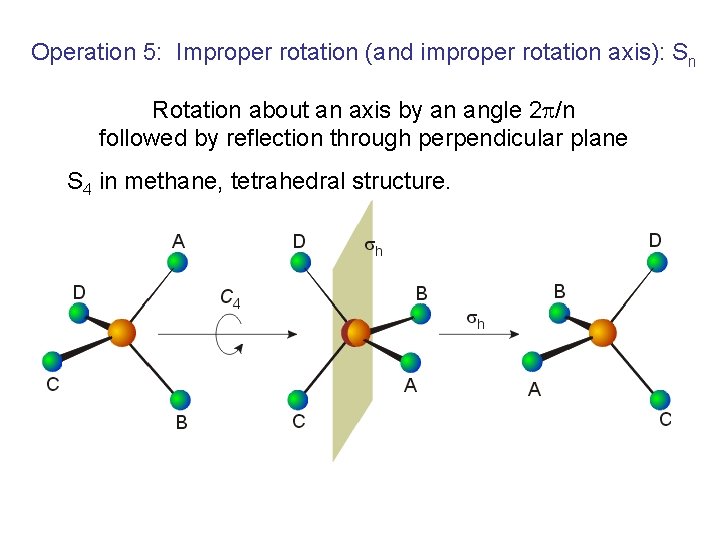
Operation 5: Improper rotation (and improper rotation axis): Sn Rotation about an axis by an angle 2 /n followed by reflection through perpendicular plane S 4 in methane, tetrahedral structure.

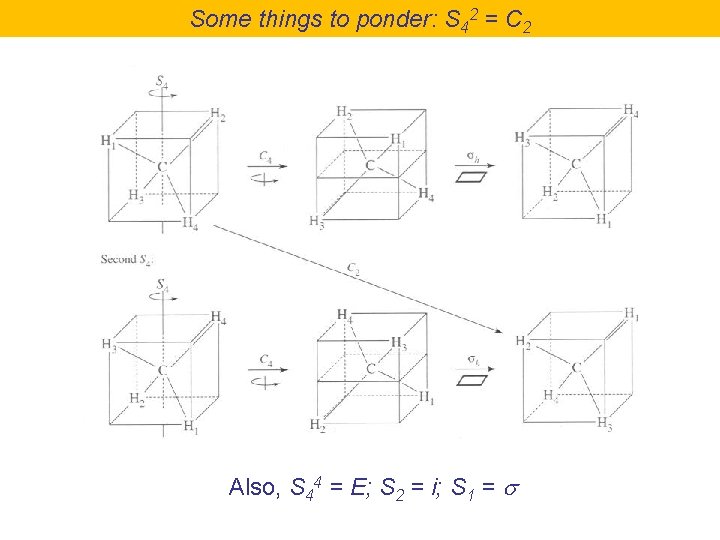
Some things to ponder: S 42 = C 2 Also, S 44 = E; S 2 = i; S 1 = s

Summary: Symmetry operations and elements Operation Element proper rotation axis (Cn) improper rotation axis (Sn) reflection plane (s) inversion center (i) Identity (E)

Successive operations, Multiplication of Operators Already talked about multiplication of rotational Operators Means m successive rotations of 2 /n each time. Total rotation is 2 m /n But let’s examine some other multiplications of operators C 4 2 1 C 4 1 4 3 C 4 We write x C 4 operators. 2 2 3 4 1 4 3 ’ = ’, first done appears to right in this relationship between

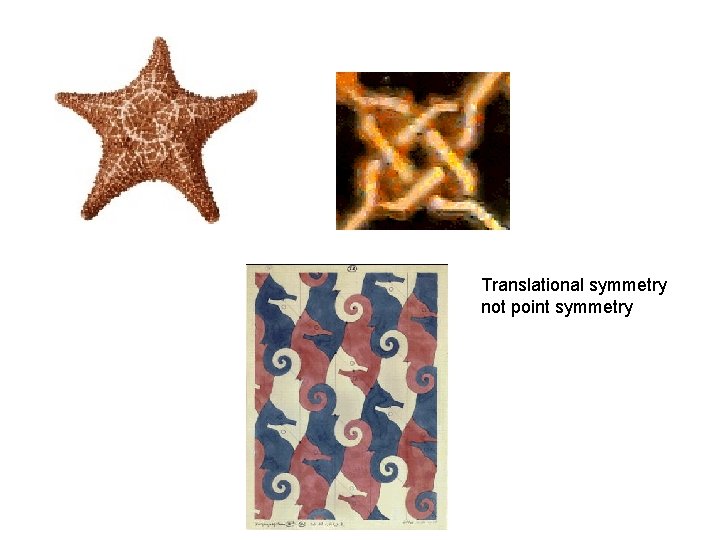
Translational symmetry not point symmetry

Symmetry point groups The set of all possible symmetry operations on a molecule is called the point group (there are 28 point groups) The mathematical treatment of the properties of groups is Group Theory In chemistry, group theory allows the assignment of structures, the definition of orbitals, analysis of vibrations, . . . See: Chemical Applications of Group Theory by F. A. Cotton

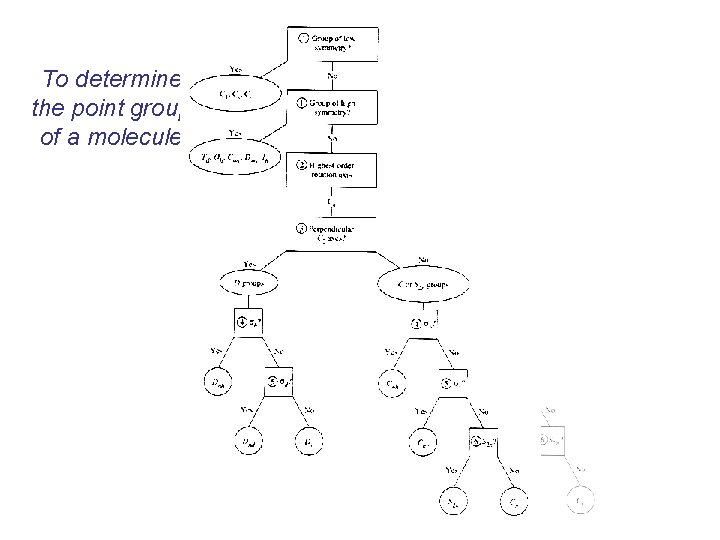
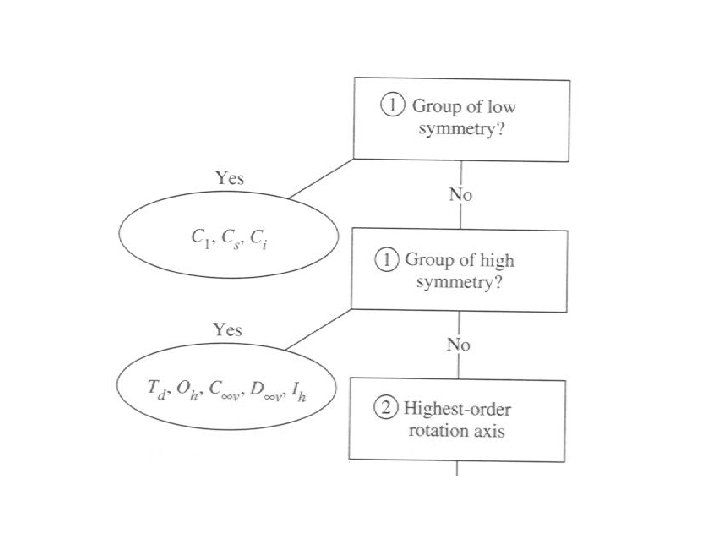
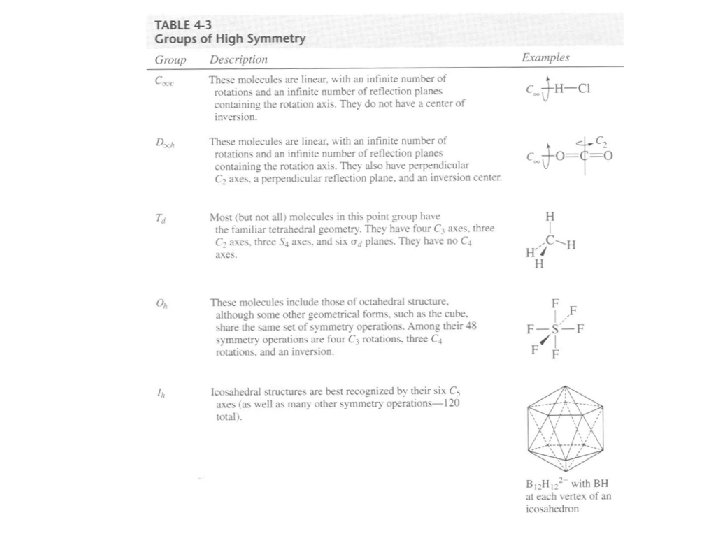
To determine the point group of a molecule


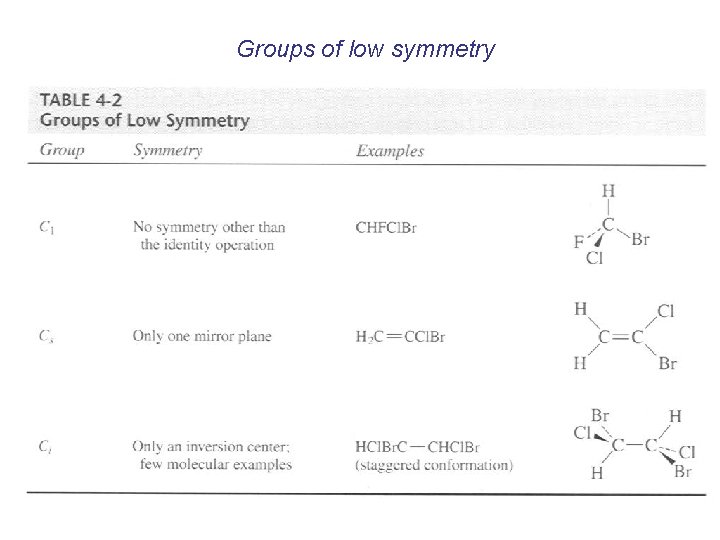
Groups of low symmetry

 Natural language processing nlp - theory lecture
Natural language processing nlp - theory lecture Natural language processing lecture notes
Natural language processing lecture notes Point group of hcl
Point group of hcl 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Joining together group theory and group skills
Joining together group theory and group skills Natural language processing lecture notes
Natural language processing lecture notes Natural language processing lecture notes
Natural language processing lecture notes Natural language processing lecture notes
Natural language processing lecture notes Natural language processing lecture notes
Natural language processing lecture notes Natural capital
Natural capital Symmetry powerpoint
Symmetry powerpoint Bayesian decision theory lecture notes
Bayesian decision theory lecture notes Sargur srihari
Sargur srihari Natural hazards vs natural disasters
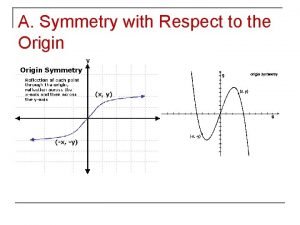
Natural hazards vs natural disasters Symmetric with respect to the x axis
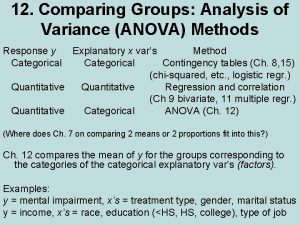
Symmetric with respect to the x axis Anova within group and between group
Anova within group and between group Classification of social group
Classification of social group Amino group and carboxyl group
Amino group and carboxyl group Amino group and carboxyl group
Amino group and carboxyl group Imt group 2 specialties
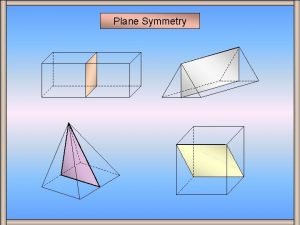
Imt group 2 specialties Plane of symmetry maths
Plane of symmetry maths Mathematics behind rubik's cube
Mathematics behind rubik's cube Brown and clough theory on group living
Brown and clough theory on group living Effective group discussion theory and practice
Effective group discussion theory and practice The social interaction source in linguistics
The social interaction source in linguistics Conscience and natural law
Conscience and natural law Chapter 15 section 1 darwins theory of natural selection
Chapter 15 section 1 darwins theory of natural selection Natural law theory
Natural law theory Natural law theory definition
Natural law theory definition Who formulated theory of evolution? *
Who formulated theory of evolution? * Jaguar natural selection
Jaguar natural selection Whats the theory of natural selection
Whats the theory of natural selection Theory of natural selection
Theory of natural selection Group polarization psychology definition
Group polarization psychology definition Y = a(b)^x
Y = a(b)^x How to compare thermal stability of group 2 nitrates
How to compare thermal stability of group 2 nitrates In group out group
In group out group Group yourself or group yourselves
Group yourself or group yourselves Sumner's classification of social groups
Sumner's classification of social groups Trait approaches to leadership
Trait approaches to leadership Continental drift vs plate tectonics theory
Continental drift vs plate tectonics theory Continental drift vs plate tectonics theory
Continental drift vs plate tectonics theory Neo classical organizational theory
Neo classical organizational theory Theory x and theory y of motivation
Theory x and theory y of motivation Semantic satiation
Semantic satiation Factors affecting column chromatography
Factors affecting column chromatography Title theory and lien theory
Title theory and lien theory X and y theory
X and y theory