Kazan State Medical University Clinical Research Centre since



- Slides: 9

Kazan State Medical University Clinical Research Centre (since 1996) Since 1994 KSMU has actively taken part in clinical medical trials in I -IV phases (certificate of accreditation for conducting clinical trials of medications for medical use № 280 of August 8, 2011).

ORGANIZATION AND CONDUCTING OF RESEARCH CLINICAL RESEARCH FOR BIOEQUIVALENCE NON-INTERVENTIONAL Post registration trials (phase 4) Clinical Research Centre offers specialized services: 1) organizing and conducting preclinical and clinical trials (I-IV phases) of any complexity in all areas of medicine at the clinical sites of KSMU; 2) preparation of documents for the conducting of clinical research; 3) accompaniment of clinical research; 4) assists contract research organizations (CRO), sponsors and clinical research departments of pharmaceutical companies in the selection of own clinical researchers and sites to conduct the medical trials.

The advantage of the organization and conducting of clinical trials at the sites of the Kazan State Medical University Ø Organization of clinical trials includes: - professional manner - highly qualified specialists - work in full compliance with the requirements of legislative framework Ø 13 partner- countries Ø 20 years of successful cooperation Ø Annually over 80 researches are conducted Ø Modern technical equipment in clinics More than 28 doctors of sciences More than 22 candidates of sciences Austria, USA, Slovenia, Denmark, Switzerland, Germany, England, France, Belgium, India, Sweden, Israel

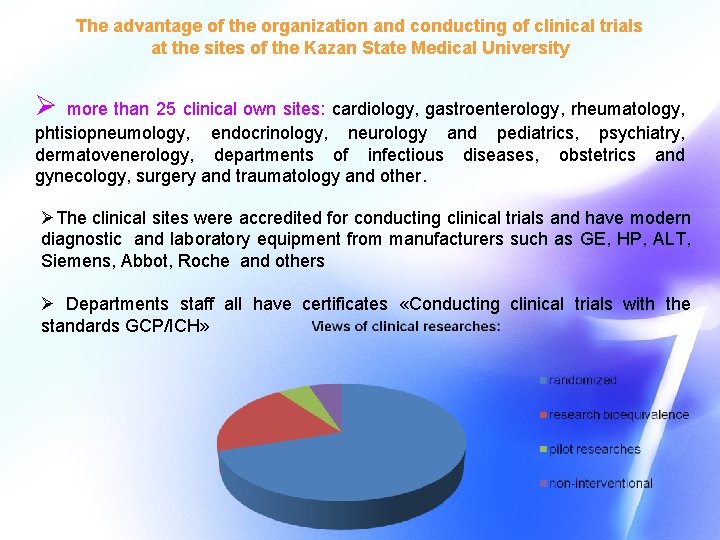
The advantage of the organization and conducting of clinical trials The advantage of the organization and conduct of clinical trials in the at the sites of the Kazan State Medical University Ø more than 25 clinical own sites: cardiology, gastroenterology, rheumatology, phtisiopneumology, endocrinology, neurology and pediatrics, psychiatry, « Worldwide Clinical Trials» dermatovenerology, departments of infectious diseases, obstetrics and gynecology, surgery and traumatology and other. «Materia Media Holding» ØThe clinical sites were accredited for conducting clinical trials and have modern «Novo Nordisk» diagnostic and laboratory equipment from manufacturers such as GE, HP, ALT, «Quintiles GMBH" Siemens, Abbot, Roche and others More than 25 CRO - partners «Medpeys» Ø Departments staff all have certificates «Conducting clinical trials with the standards GCP/ICH» «RUSS» «Atoll» «Parexel» F. Hoffman – La Roche Ltd «Krka d. d. »

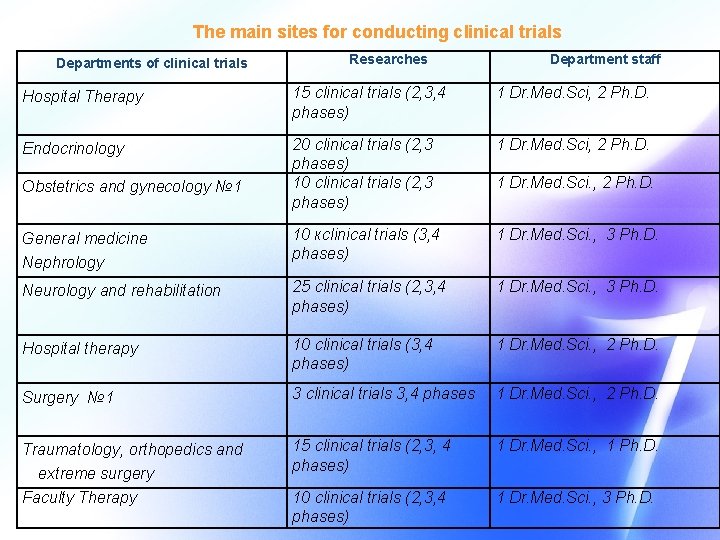
The main sites for conducting clinical trials Departments of clinical trials Researches Department staff Hospital Therapy 15 clinical trials (2, 3, 4 phases) 1 Dr. Med. Sci, 2 Ph. D. Endocrinology 20 clinical trials (2, 3 phases) 1 Dr. Med. Sci, 2 Ph. D. General medicine Nephrology 10 кclinical trials (3, 4 phases) 1 Dr. Med. Sci. , 3 Ph. D. Neurology and rehabilitation 25 clinical trials (2, 3, 4 phases) 1 Dr. Med. Sci. , 3 Ph. D. Hospital therapy 10 clinical trials (3, 4 phases) 1 Dr. Med. Sci. , 2 Ph. D. Surgery № 1 3 clinical trials 3, 4 phases 1 Dr. Med. Sci. , 2 Ph. D. Traumatology, orthopedics and extreme surgery Faculty Therapy 15 clinical trials (2, 3, 4 phases) 1 Dr. Med. Sci. , 1 Ph. D. 10 clinical trials (2, 3, 4 phases) 1 Dr. Med. Sci. , 3 Ph. D. Obstetrics and gynecology № 1 1 Dr. Med. Sci. , 2 Ph. D.

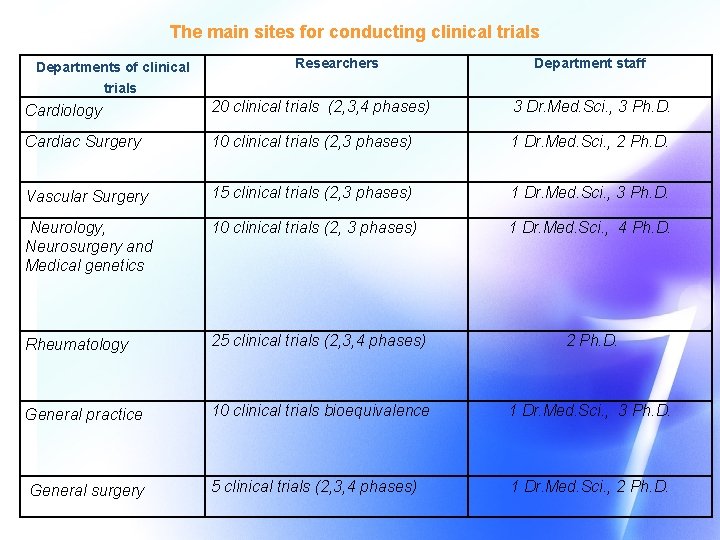
The main sites for conducting clinical trials Departments of clinical Researchers Department staff trials Cardiology 20 clinical trials (2, 3, 4 phases) 3 Dr. Med. Sci. , 3 Ph. D. Cardiac Surgery 10 clinical trials (2, 3 phases) 1 Dr. Med. Sci. , 2 Ph. D. Vascular Surgery 15 clinical trials (2, 3 phases) 1 Dr. Med. Sci. , 3 Ph. D. Neurology, Neurosurgery and Medical genetics 10 clinical trials (2, 3 phases) 1 Dr. Med. Sci. , 4 Ph. D. Rheumatology 25 clinical trials (2, 3, 4 phases) 2 Ph. D. General practice 10 clinical trials bioequivalence 1 Dr. Med. Sci. , 3 Ph. D. General surgery 5 clinical trials (2, 3, 4 phases) 1 Dr. Med. Sci. , 2 Ph. D.

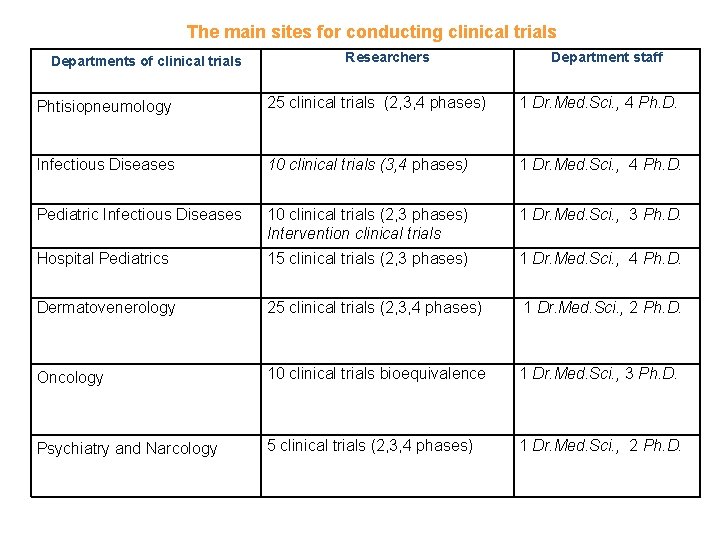
The main sites for conducting clinical trials Departments of clinical trials Researchers Department staff Phtisiopneumology 25 clinical trials (2, 3, 4 phases) 1 Dr. Med. Sci. , 4 Ph. D. Infectious Diseases 10 clinical trials (3, 4 phases) 1 Dr. Med. Sci. , 4 Ph. D. Pediatric Infectious Diseases 10 clinical trials (2, 3 phases) Intervention clinical trials 1 Dr. Med. Sci. , 3 Ph. D. Hospital Pediatrics 15 clinical trials (2, 3 phases) 1 Dr. Med. Sci. , 4 Ph. D. Dermatovenerology 25 clinical trials (2, 3, 4 phases) 1 Dr. Med. Sci. , 2 Ph. D. Oncology 10 clinical trials bioequivalence 1 Dr. Med. Sci. , 3 Ph. D. Psychiatry and Narcology 5 clinical trials (2, 3, 4 phases) 1 Dr. Med. Sci. , 2 Ph. D.

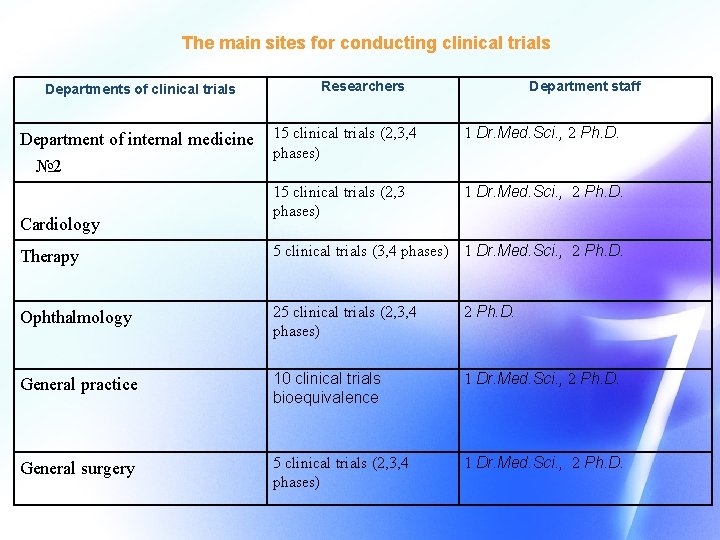
The main sites for conducting clinical trials Departments of clinical trials Department of internal medicine № 2 Cardiology Researchers Department staff 15 clinical trials (2, 3, 4 phases) 1 Dr. Med. Sci. , 2 Ph. D. 15 clinical trials (2, 3 phases) 1 Dr. Med. Sci. , 2 Ph. D. Therapy 5 clinical trials (3, 4 phases) 1 Dr. Med. Sci. , 2 Ph. D. Ophthalmology 25 clinical trials (2, 3, 4 phases) 2 Ph. D. General practice 10 clinical trials bioequivalence 1 Dr. Med. Sci. , 2 Ph. D. General surgery 5 clinical trials (2, 3, 4 phases) 1 Dr. Med. Sci. , 2 Ph. D.

Clinical research centre invites you to cooperation! Contact information: E-mail: cki_kgmu@mail. ru тел. 8 (843) 236 -74 -92 Site: http: //kgmu. kcn. ru/science-and-innovation/innova. html















