IWGT Group 4 Improving in vivo genotoxicity testing

- Slides: 14

IWGT Group 4 Improving in vivo genotoxicity testing- the link to standard toxicity testing Summary of discussion items, conclusions and recommendations Hans-Joerg Martus Novartis Institutes for Bio. Medical Research Basel, Switzerland

IWGT Working Group 4 • Andreas Rothfuss (Chair) • Masa Honma (Co-chair) • Hans-Joerg Martus (Rapporteur) • Marilyn Aardema • Brian Burlinson • Andreas Czich • Sheila Galloway • Shuichi Hamada • Bob Heflich 2 • • • Jon Howe Peter Kasper David Kirkland Makoka Nakajima Mike O‘Donovan Ulla Plappert-Helbig Les Recio Maik Schuler Yoshifumi Uno Group 4: Integration of genotoxicity into routine studies 2

Topics to be addressed 1. Combination of MN assay and Comet Assay into acute studies 2. Integration of the MNT into repeated-dose toxicity (RDT) studies 3. Integration of the Comet Assay into RDT studies 4. Requirements on top dose for „integrated“ RDT studies 5. Further tests suitable for integration (not covered due to time constraints) 3 Group 4: Integration of genotoxicity into routine studies 3

Improving in vivo genotoxicity testing • Animal welfare recommendations to reduce animal usage – Reduce “false positive” rate in vitro – Apply smarter testing strategies in vivo • Options for improvement in vivo: Combination and Integration – Combination of acute assays into one stand-alone study. – Integration of genotoxicity endpoints into repeat dose toxicity (RDT) studies • Chances for Improvement beyond 3 R’s – Improved genotoxicity risk assessment: Interpretation of genotoxicity results in conjunction with toxicity data and toxicokinetics – More efficient testing using new technologies – Potential for resource savings (compound, manpower) 4 Group 4: Integration of genotoxicity into routine studies 4

Current Recommendations • Linking genotoxicity tests to General Toxicity is not a new concept: Integration of MN assays has been discussed since a while now… – EMS workshop, Mac. Gregor et al. , EMM 25: 328 -337, 1995 – IWGT, Hayashi et al, EMM 35: 234 -252, 2000 - More recent guidances: - REACh ITS (Integrated Testing Strategy) • Integration of genotoxicity tests (e. g. MN assay, Comet Assay) into repeat -dose tox studies, if scientifically justified – ICH S 2(R) draft guidance • Integration of in vivo genotoxicity endpoints into RDT studies preferable, if scientifically justified • If more than one genotoxicity endpoint, incorporation into a single study is preferred. – ECVAM workshop on Animal reduction (Pfuhler et al. , submitted) • Integration of MN assay into RDT studies “should be standard if RDT studies are foreseen for the test compound”. • Combination of acute MN and Comet Assay studies into one study. 5 Group 4: Integration of genotoxicity into routine studies 5

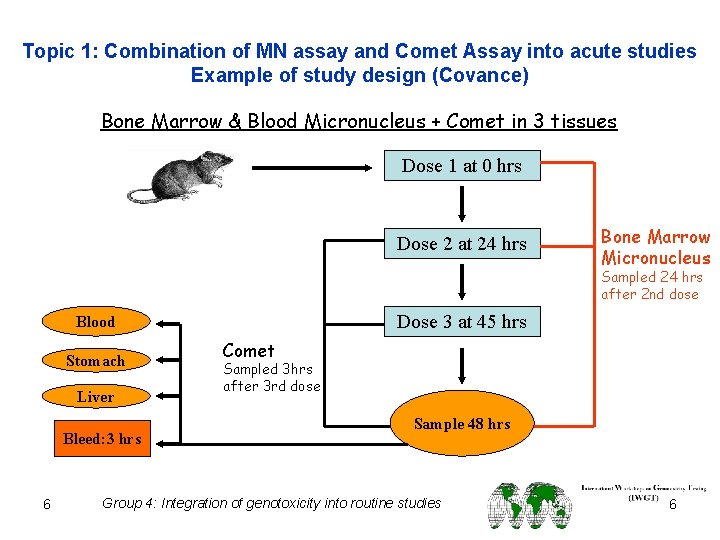
Topic 1: Combination of MN assay and Comet Assay into acute studies Example of study design (Covance) Bone Marrow & Blood Micronucleus + Comet in 3 tissues Dose 1 at 0 hrs Dose 2 at 24 hrs Bone Marrow Micronucleus Sampled 24 hrs after 2 nd dose Dose 3 at 45 hrs Blood Stomach Liver Bleed: 3 hrs 6 Comet Sampled 3 hrs after 3 rd dose Sample 48 hrs Group 4: Integration of genotoxicity into routine studies 6

Topic 1: Combination of MN assay and Comet Assay into acute studies Consensus statements: – Combination of MNT and comet technically feasible and scientifically acceptable as an alternative to the separate assays • Promising results obtained with (mostly) model compounds • 3 d or 4 d protocols equally acceptable 7 Group 4: Integration of genotoxicity into routine studies 7

Topic 2: Integration of MNT into repeat-dose toxicology (RDT) studies (I) Consensus statements: • Integration of MNT into RDT is scientifically acceptable • There may be situations (e. g. severe bone marrow toxicity) where an acute study is preferable 8 Group 4: Integration of genotoxicity into routine studies 8

Topic 2: Integration of MNT into repeated-dose toxicology (RDT) studies (II) Consensus statements on „Additional early peripheral blood sampling time point on day 4“ – Early sampling not routinely required but can help in study evaluation if data show unsuitability of late sampling time point – If result from terminal sampling is negative and marked myelotoxicity is evident then the additional early sampling timepoint may provide useful data – Early sampling can be advisable to investigate erythropoiesisrelated effects 9 Group 4: Integration of genotoxicity into routine studies 9

Topic 2: Integration of MNT into repeated-dose toxicology (RDT) studies (III) Consensus statements on „Effect of Bleeding“ – Animals bled for TK or other purposes can be used for MN analysis • For rats above 9 weeks the current data suggest that bleeding might not affect response to genotoxins as long as MN frequencies in control animals are unaffected • One example exists to suggest that for rats aged 5 weeks bleeding might affect the MN response • It it advisable to use minimal volumes and low frequencies when withdrawing blood to minimize disturbance of erythropoiesis – No data to indicate generation of false-positive results in rats 10 Group 4: Integration of genotoxicity into routine studies 10

Topic 3: integration of comet into RDT studies Consensus statements • Integration of comet into RDT studies – Integration into RDT is considered scientifically acceptable – Liver comet assay complements MNT in blood or bone marrow in detecting in vivo genotoxins – Practical issues need to be considered • Cytotoxicity – Data available so far indicate that cytotoxicity does not generate increases or decreases in DNA migration 11 Group 4: Integration of genotoxicity into routine studies 11

Topic 4: Top dose in RDT study Background information: Arguments discussed within ICHS 2 revision • Criteria to qualify a repeated dose test (≥ 2 weeks) as acceptable for option 2 (no or positive in vitro mammalian test): – – – • Maximum feasible dose (formulation) Exposure plateau Limit dose: 1000 mg/kg Accumulation with repeated dosing At least 50% of acute MTD For aneugens and certain hematotoxic clastogens: – 2 -4 day blood sampling from the multiweek study before substantial hematotoxicity develops – Acute in vivo test Criteria to disqualify a repeated dose test for option 2: – MTD alone – Reduced exposure to parent drug with time ( 50%) (in that sex) • Typically seen in rats, especially males, and attributable to enzyme induction; not necessarily relevant to human; prevents maximal exposure to parent, although it does provide exposure to metabolite 12 Group 4: Integration of genotoxicity into routine studies 12

Topic 4: Top dose in RDT study Consensus statements • MTD considered acceptable for in vitro negatives • For in vitro positives (or no in vitro data) MTD is acceptable in many cases such as: – RDT MTD (or exposure) close (up to two-fold) to acute MTD (or exposure) – Estimated human exposure is lower by a large margin • If deviating therefrom use proper justification 13 Group 4: Integration of genotoxicity into routine studies

Group 4: Overall conclusions • Combination of MNT and comet is scientifically justified for both acute and RDT studies • Most recommendations are based on a limited data set and need to be refined in the future • Future prospects: – Evaluate genotoxins with diverse modes-of-action – Evaluate compounds with extrahepatic target tissues (comet) – Consider involving NTP for future experiments 14 Group 4: Integration of genotoxicity into routine studies 14