IONS Ions Charged particles which form when an










- Slides: 13

IONS

Ions • Charged particles which form when an atom (or group of atoms) gains or loses electrons.

Ions • Charged particles which form when an atom (or group of atoms) gains or loses electrons. • Ions don’t have equal number of PROTONS and ELECTRONS

Ions • Charged particles which form when an atom (or group of atoms) gains or loses electrons. • Ions don’t have equal number of PROTONS and ELECTRONS • Each ion (or group) is represented by a symbol which shows the charge of the atom

Ions • Charged particles which form when an atom (or a group of atoms) gains or loses electrons. • Ions don’t have equal number of PROTONS and ELECTRONS • Each ion (or group) is represented by a symbol which shows the charge of the atom • Metals form positively charged ions while non-metals may form negatively charged ions.

Ions • Charged particles which form when an atom (or a group of atoms) gains or loses electrons. • Ions don’t have equal number of PROTONS and ELECTRONS • Each ion (or group) is represented by a symbol which shows the charge of the atom • Metals form positively charged ions while non-metals may form negatively charged ions. • EG. Na+ is a sodium ion. The + means the charge is +1. This means it has LOST one electron.

Ions • Charged particles which form when an atom (or a group of atoms) gains or loses electrons. • Ions don’t have equal number of PROTONS and ELECTRONS • Each ion (or group) is represented by a symbol which shows the charge of the atom • Metals form positively charged ions while non-metals may form negatively charged ions. • EG. Na+ is a sodium ion. The + means the charge is +1. This means it has LOST one electron • EG. O 2 - is an oxide ion. The 2 - sign means it has 2 more electrons than protons

Ions • Cations – positively charged ions that have lost electrons.

Ions • Cations – positively charged ions that have lost electrons. • Anions – negatively charged ions which have gained electrons.

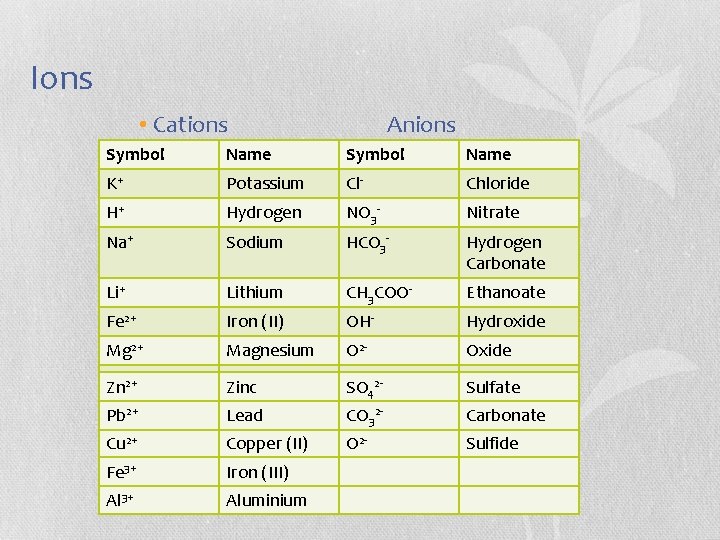
Ions • Cations Anions Symbol Name K+ Potassium Cl- Chloride H+ Hydrogen NO 3 - Nitrate Na+ Sodium HCO 3 - Hydrogen Carbonate Li+ Lithium CH 3 COO- Ethanoate Fe 2+ Iron (II) OH- Hydroxide Mg 2+ Magnesium O 2 - Oxide 2 Ca Zn 2++ Calcium Zinc 2 S SO- 42 - Sulfate Pb 2+ Lead CO 32 - Carbonate Cu 2+ Copper (II) O 2 - Sulfide Fe 3+ Iron (III) Al 3+ Aluminium

Questions: 1. The chloride ion has 18 electrons and is charged -1. a) Write the symbol for the chloride ion b) How many protons has the chloride ion got? 2. The magnesium ion has 12 protons and is charged +2 a) Write down its symbol b) Decide how many electrons the magnesium ion has

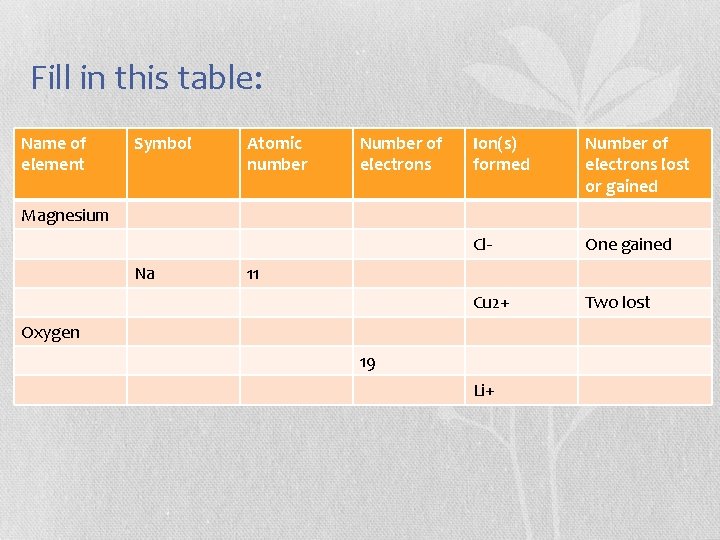
Fill in this table: Name of element Symbol Atomic number Number of electrons Ion(s) formed Number of electrons lost or gained Cl- One gained Cu 2+ Two lost Magnesium Na 11 Oxygen 19 Li+

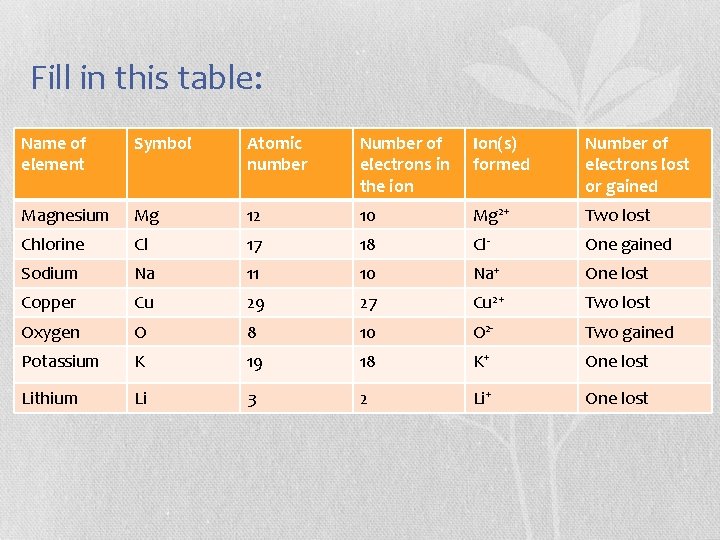
Fill in this table: Name of element Symbol Atomic number Number of electrons in the ion Ion(s) formed Number of electrons lost or gained Magnesium Mg 12 10 Mg 2+ Two lost Chlorine Cl 17 18 Cl- One gained Sodium Na 11 10 Na+ One lost Copper Cu 29 27 Cu 2+ Two lost Oxygen O 8 10 O 2 - Two gained Potassium K 19 18 K+ One lost Lithium Li 3 2 Li+ One lost