Ions Ions Charged particles Atoms that have more




- Slides: 15
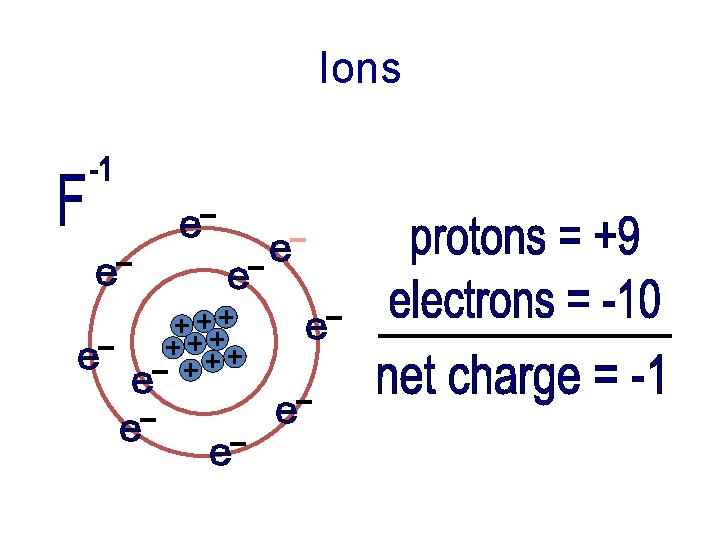
Ions

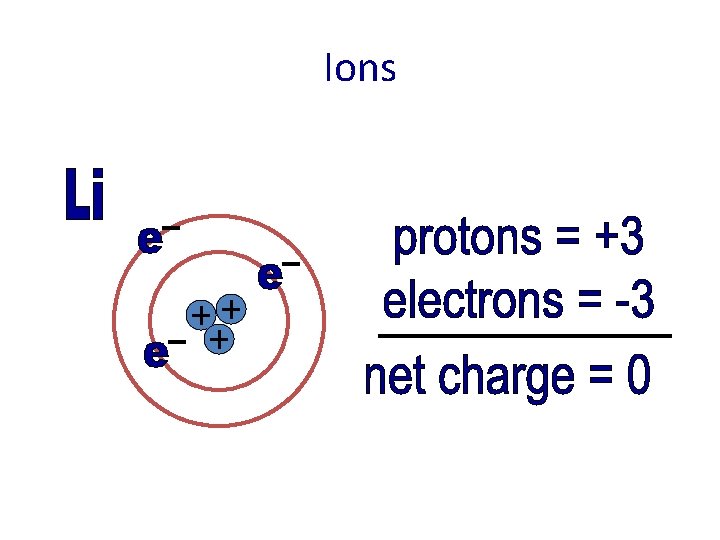
Ions • Charged particles • Atoms that have more or less electrons than protons • Ions try to imitate the nearest stable element

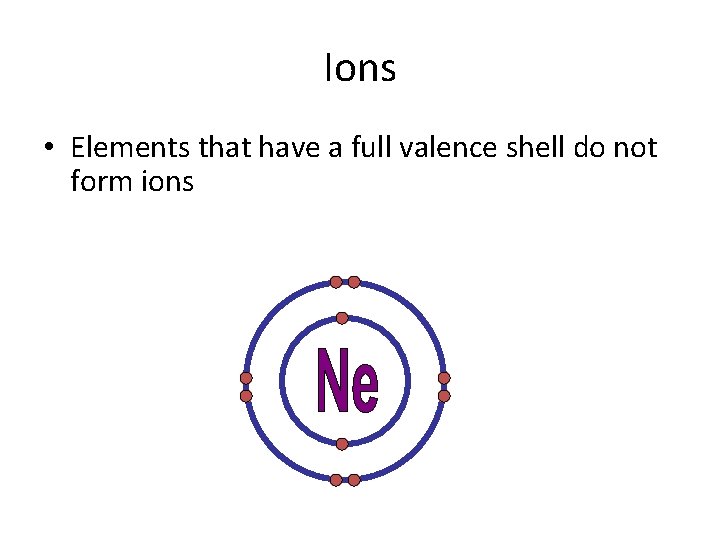
Ions • Elements that have a full valence shell do not form ions

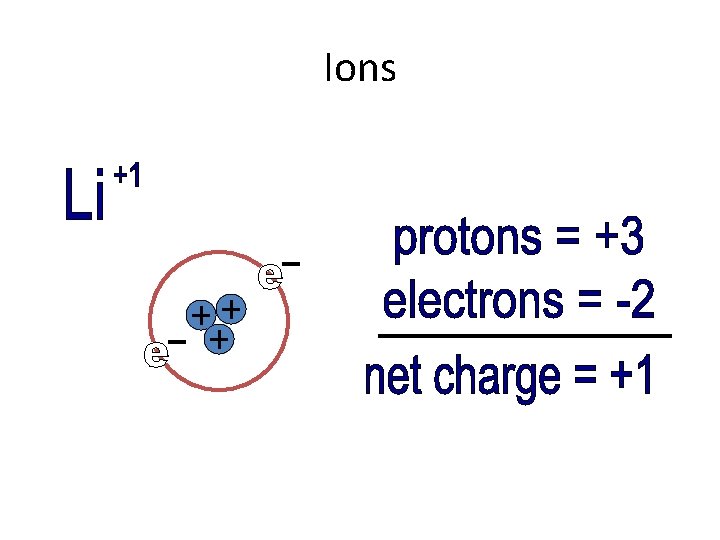
Ions • Elements that have one, two, or three valence electrons tend to lose electrons to become stable • If an atom loses electrons (which are negative), there are more protons. This means that the atom becomes positive.

Ions

Ions

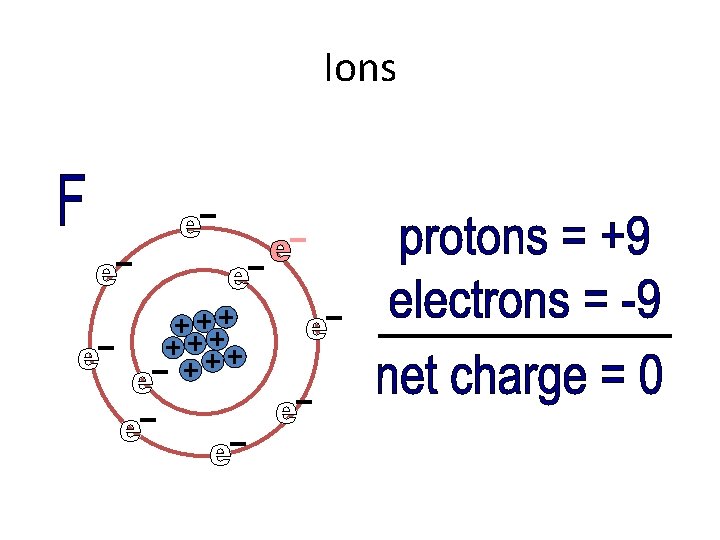
Ions • Elements that have five, six, or seven valence electrons try to gain electrons to become stable • If an atom gains electrons (which are negative), there are fewer protons. So the atom becomes negative.

Ions

Ions

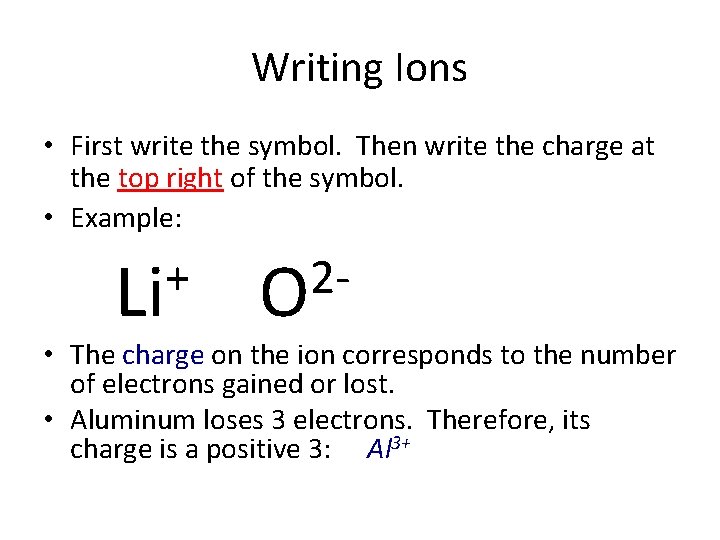
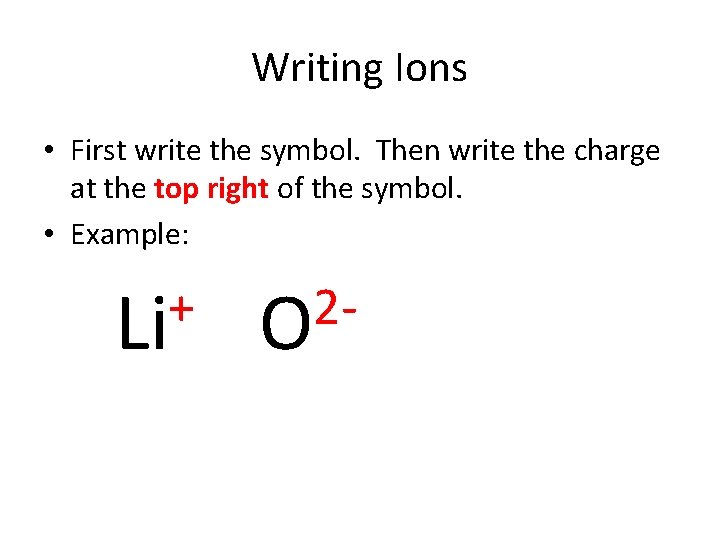
Writing Ions • First write the symbol. Then write the charge at the top right of the symbol. • Example: + Li 2 O • The charge on the ion corresponds to the number of electrons gained or lost. • Aluminum loses 3 electrons. Therefore, its charge is a positive 3: Al 3+

Writing Ions • First write the symbol. Then write the charge at the top right of the symbol. • Example: + Li 2 O

Superscript This is the number written toward the top of a chemical symbol to indicate what the oxidation number (charge) on that atom is. When there is no superscript, we assume the charge is zero.

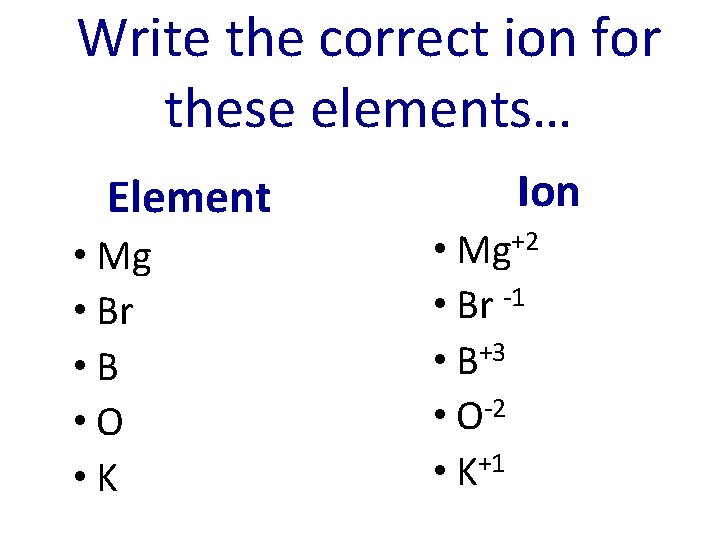
Write the correct ion for these elements… Element • Mg • Br • B • O • K Ion • Mg+2 • Br -1 • B+3 • O-2 • K+1

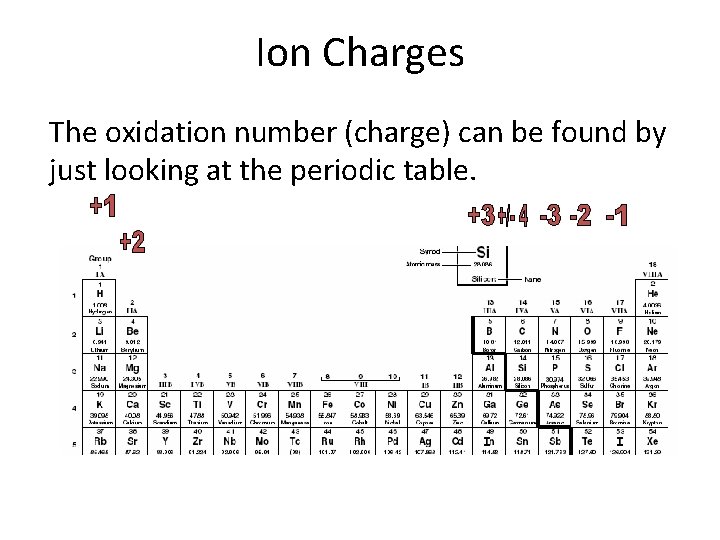
Ion Charges The oxidation number (charge) can be found by just looking at the periodic table.

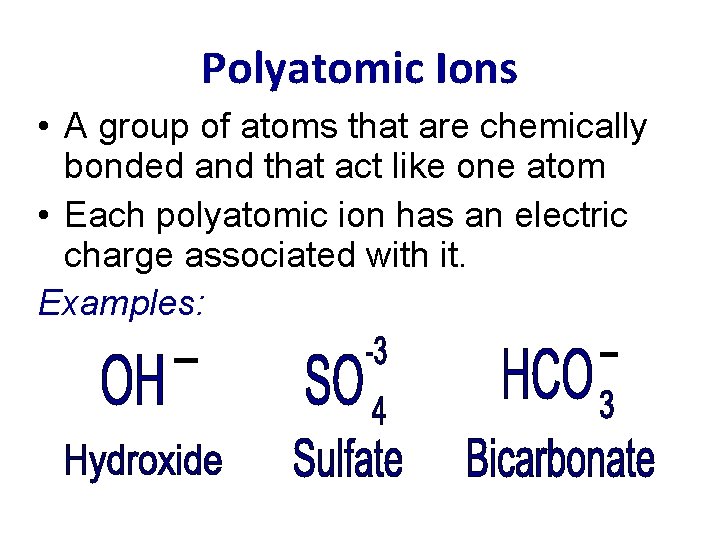
Polyatomic Ions • A group of atoms that are chemically bonded and that act like one atom • Each polyatomic ion has an electric charge associated with it. Examples:
 More more more i want more more more more we praise you
More more more i want more more more more we praise you More more more i want more more more more we praise you
More more more i want more more more more we praise you Chapter 6 ions charged particles in solution
Chapter 6 ions charged particles in solution Positive ions are atoms that have
Positive ions are atoms that have The search for fractionally charged particles has
The search for fractionally charged particles has Charged particles can be accelerated by
Charged particles can be accelerated by For charged particles, what is the quantity q/m called?
For charged particles, what is the quantity q/m called? Gas like mixture of charged particles
Gas like mixture of charged particles Properties of ionic compounds
Properties of ionic compounds Atoms are small, hard particles
Atoms are small, hard particles Atoms seldom exist as independent particles
Atoms seldom exist as independent particles Atoms seldom exist as independent particles
Atoms seldom exist as independent particles Lewis structure for c2hcl
Lewis structure for c2hcl How are the particles that make up atoms diagrammed
How are the particles that make up atoms diagrammed Triselenium octafluoride formula
Triselenium octafluoride formula Regents periodic table
Regents periodic table