Investigational Devices and Humanitarian Use Devices June 2007



























- Slides: 28

Investigational Devices and Humanitarian Use Devices June 2007

Investigational device n Any healthcare product that does not achieve its primary intended purpose by chemical action or by being metabolized.

Devices n Investigational Device Exemption (IDE) – A FDA approved investigational device exemption permits a device to be shipped lawfully for the purpose of conducting investigations

Significant Risk (SR) Device Study A study of a device that presents a potential for serious risk to the health, safety, or welfare of a participant n–

Non-significant Risk (NSR) Device Study n. A study of a device that does not meet the definition of a SR device and does not present a potential for serious risk to the health, safety, or welfare of participants

Devices – Investigator responsibilities (Document 53) n The investigator must provide the IRB with: – All available information regarding the use of the device, including IDE #, if applicable. – When IDE is required, PI must complete FDA’s Investigator agreement form and send copy. – All correspondence from the sponsor and/or FDA regarding if SR or NSR

Investigator responsibilities – – – Sponsor’s report of prior investigation Notify sponsor of the SR decision of the IRB Describe the ingredients, properties, etc of device. Complete informed consent forms Maintain all case report forms Be accountable for storage, dispensing, tracking, and oversight of device – Submit continuing reviews, adverse events, or unanticipated problems, and clinical outcomes of each participant, if known.

Device – PI responsibility – Notify IRB of any amendments, unanticipated device effects, serious adverse events, or unanticipated problems. Notify IRB of study closure Return all unused products per sponsor’s instructions Submit final report within three months of termination or completion of study.

IRB responsibilities n Both SR and NSR studies must go to full board for initial review and approval. – Must determine if SR or NSR (regardless of sponsor’s determination) – Once determination made, IRB decides whether to approve the study. – Approval based on same criteria as any other FDA regulated study

IRB responsibilities IRB will determine whether investigator is knowledgeable about regulatory requirements and may require PI (and staff) to attend training sessions designed and conducted by the QA specialist. n This is particularly true if WVU or an individual assumes the sponsor function. n

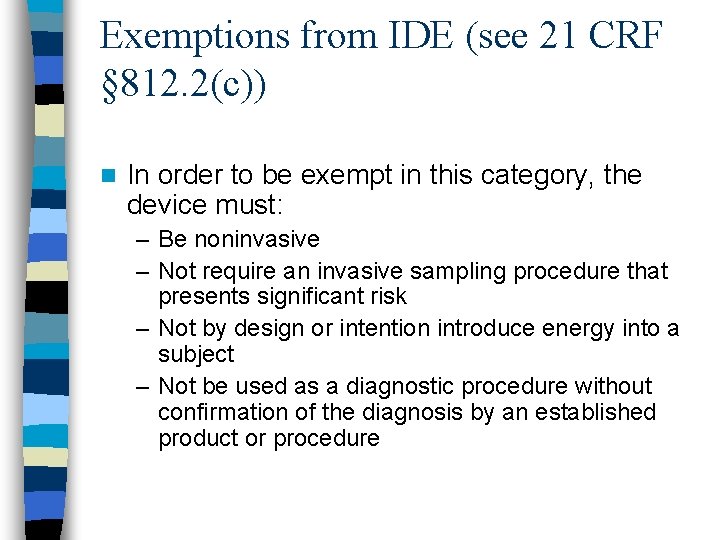
Exemptions from IDE requirements n. A device can be exempt from IDE requirements – a claim for exemption must reference the exemption category

Exemptions from IDE (see 21 CRF § 812. 2(c)) n In order to be exempt in this category, the device must: – Be noninvasive – Not require an invasive sampling procedure that presents significant risk – Not by design or intention introduce energy into a subject – Not be used as a diagnostic procedure without confirmation of the diagnosis by an established product or procedure

Another exemption category (21 CFR § 812(c)(4)) n To qualify under this category, the device must not be for the purpose of determining safety and effectiveness and must not put subjects at risk. It must be limited to: – Consumer preference testing – Testing of a modification; or – Testing of a combination of two or more devices in commercial distribution.

Advertising or recruitment n Must not include the term “new treatment” without explaining that the IDE is “investigational, meaning non. FDA approved” n Must not include the promise of “free medical treatment”

Informed consent and additional requirements n Informed consent with IDEs must meet requirements of the IRB policies and procedures. – There must be no claims of safety or superiority over other devices. – Must be a statement that the IDE is “investigational”, that is non-FDA approved. – The FDA must have access to subject’s medical records. – The participant must know that the IDE is experimental and benefits are unproven.

Informed consent and additional requirements n Any unanticipated adverse device effect to participants or other must be reported to the IRB no later than 10 working days after the PI learns of the event.

Humanitarian Use Devices n Humanitarian Use Device (HUD)- a device that is intended to be used in fewer than 4, 000 individual in the United States per year. n Humanitarian Use Device Exemption (HDE)- an FDA approval for a physician to use a HUD in clinical treatment or as the subject of a clinical investigation.

IRB responsibilities n An HDE must be issued by the FDA in order for a HUD to be used in treatment, diagnosis, or research at WVU – The IRB approval must verify that the use of the HUD is congruent with current labeling of the device and does not exceed the scope of the FDA indication. – The IRB may impose more stringent restrictions, if it feels they are necessary

IRB requirements (HUD) The initial review must be full-board (IRB may determine that expedited review at subsequent continuing reviews is adequate) n The IRB will not require informed consent since the use is not considered research. The IRB, however, requires that the patient be informed of the use by a cover letter. n The IRB will establish that the investigator and research team are trained; it not the IRB will require that PI and research team receive training on regulatory requirements for HUD by QA specialist. n

IRB responsibilities At the present time, the IRB is requiring that a continuing protocol must be submitted at three-month intervals. n The continuing request must discuss: n – the number of times the HUD has been used; – any problems associated with its use – any information received from the sponsor or the FDA concerning the use of the HUD at other sites.

IRB responsibilities n The report must also provide a chronological listing of all uses n A copy of the hospital consent that indicates this is for an HUD n A copy of the note in the patient’s chart stating that the patient received the cover letter.

HUD – Investigator’s responsibilities n The physician must use the HUD only in accordance with the labeling of the device and in the designated population of FDA approval. n Only the holder of the HDE agreement with the FDA must use the HUD

PI responsibilities Must report any unanticipated problems or any information that the HUD contributed to the death or serious injury of a patient to the FDA and the IRB promptly. n Report any FDA actions to the IRB n Report any modifications to HUD or its clinical use to the IRB n Notify the patient(s) of any new information concerning the use and safety of the HUD n

PI responsibilities n Prepare a cover letter to be given to patients receiving a HUD – Explain a HUD and what that means – List all risks and procedures associated with the use of the HUD – Inform the patient that the device is approved only for certain uses and that the patient meets the conditions of its intended use. – Inform the patient that the costs will be charged to the patient or the patient’s insurance

Emergency use of FDA regulated devices n The use of an investigational device with a human subject in an immediate serious life-threatening situation in which no standard acceptable treatment is available and in which there is not sufficient time to obtain IRB approval.

Procedure n If it is anticipated that a device might be used in an emergency use situation, and there is sufficient time, the physician should provide sufficient information to the IRB so that it could determine if the anticipated use meets the FDA regulatory requirements.

Procedure However, emergency use usually takes place without time for IRB review. The physician should: Obtain concurrence of IRB Chair if time permits. Obtain informed consent from the patient or legal representative, if possible. Obtain independent assessment by an uninvolved physician. Obtain institutional clearance n

Procedure after use: n Should notify the holder of the IDE or HDE. n The use must be reported to the IRB within 5 working days. n Information on use and patient’s condition to the holder of the IDE or HDE, who will make a report to the FDA.
 Investigational product manufacturing
Investigational product manufacturing June 2007 physics regents
June 2007 physics regents Ee.humanitarian response.info/x/vpdlixjf
Ee.humanitarian response.info/x/vpdlixjf Humanitarian work psychology
Humanitarian work psychology Humanitarian projects in prague for youth
Humanitarian projects in prague for youth Wcag
Wcag Humanitarian programme cycle 2021
Humanitarian programme cycle 2021 Cadet humanitarian award
Cadet humanitarian award Afjrotc distinguished cadet badge
Afjrotc distinguished cadet badge Humanitarian engineers in cape york
Humanitarian engineers in cape york International humanitarian law icrc
International humanitarian law icrc International humanitarian law icrc
International humanitarian law icrc Ihl
Ihl Khalifa humanitarian foundation
Khalifa humanitarian foundation Sphere book humanitarian
Sphere book humanitarian Humanitarian work psychology
Humanitarian work psychology 30 days has september
30 days has september February march april may june
February march april may june How you use ict today and how you will use it tomorrow
How you use ict today and how you will use it tomorrow Literary elements and definitions
Literary elements and definitions Scanner is input or output device
Scanner is input or output device Spanish flacs exam 2018 answers
Spanish flacs exam 2018 answers June 2005 calendar
June 2005 calendar To a daughter leaving home and poem for my sister
To a daughter leaving home and poem for my sister Good morning 1 june
Good morning 1 june Foreshadowing in the lottery
Foreshadowing in the lottery Danswer
Danswer Welcome june blessings
Welcome june blessings English language paper 1 june 2021
English language paper 1 june 2021