Introduction to DYNAMIC LIGHT SCATTERING DLS or PHOTON







![Arrhenius Plot log[ Relaxation time (s)] 4 Polymer (n=69) Dimer (n=2) 0 Oligomer (n=7) Arrhenius Plot log[ Relaxation time (s)] 4 Polymer (n=69) Dimer (n=2) 0 Oligomer (n=7)](https://slidetodoc.com/presentation_image_h/7f248dbc6b9c1f3c54e3fc60097e89cc/image-21.jpg)


- Slides: 31

Introduction to DYNAMIC LIGHT SCATTERING (DLS) or PHOTON CORRELATION SPECTROSCOPY (PCS) Christer Svanberg

Outline Basic of DLS – Experimental set-up – Accessible time- and length-scales Applications – Size and shape of sub-micron objects Research – Glass transition – Polymer dynamics

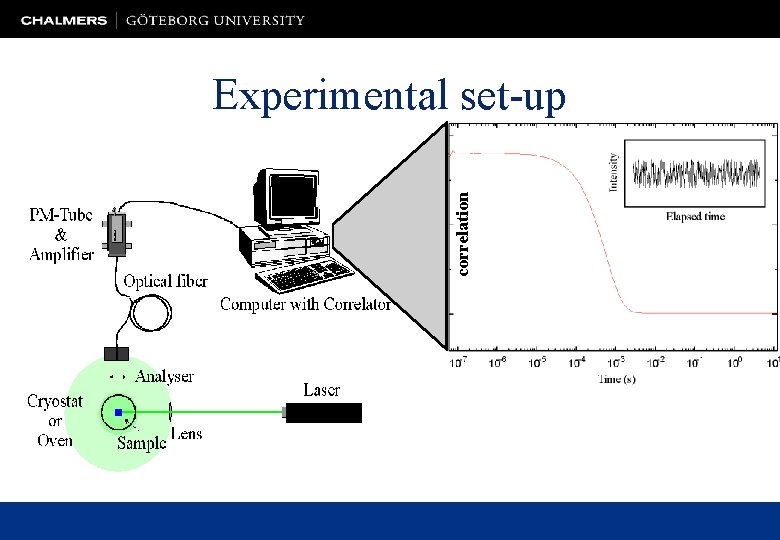
correlation Experimental set-up

Scattered Electric Field DLS probes • density fluctuations • concentration fluctuations

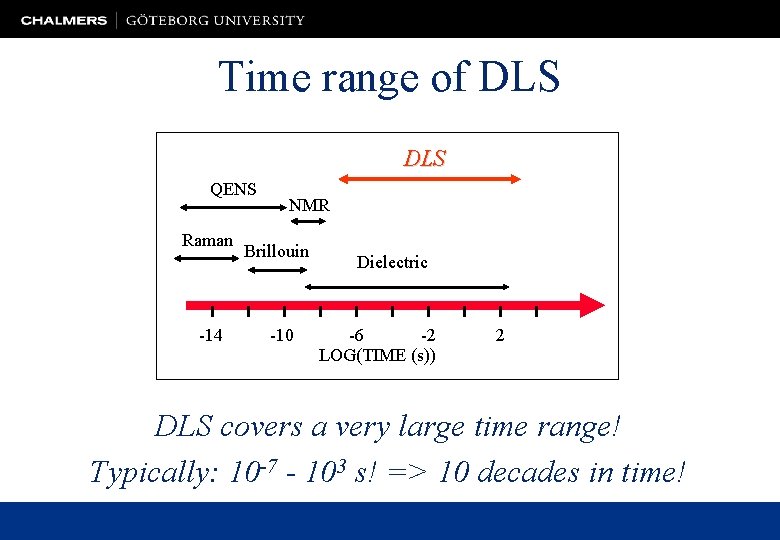
Time range of DLS QENS Raman -14 NMR Brillouin -10 Dielectric -6 -2 LOG(TIME (s)) 2 DLS covers a very large time range! Typically: 10 -7 - 103 s! => 10 decades in time!

Experimentally accessible wave vectors Q-range: typically 0. 6 – 2× 10 -3 Å-1 DLS is therefore suitable for diffusional studies of macromolecules, such as polymers and large biomolecules!

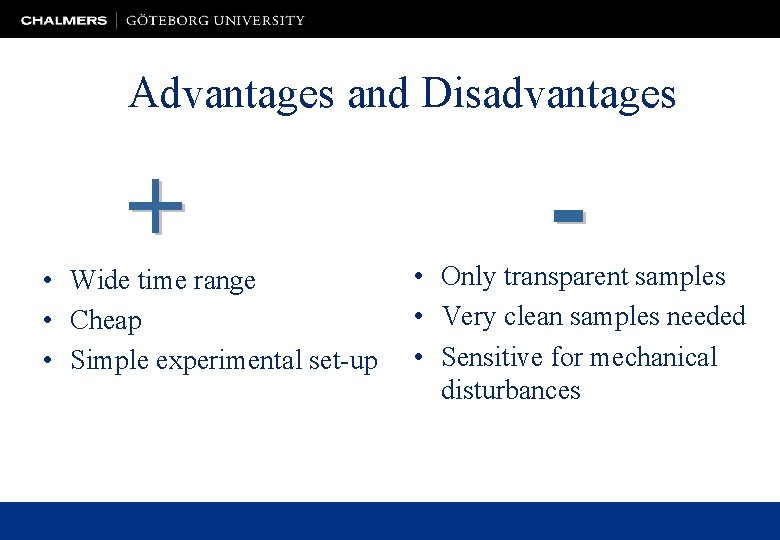
Advantages and Disadvantages • Wide time range • Cheap • Simple experimental set-up • Only transparent samples • Very clean samples needed • Sensitive for mechanical disturbances

Commerically avaliable products

Scientific instruments • • More laser power! Specially designed cryo-furnaces Polarization options Vibration isolation table

Scotland, 1827

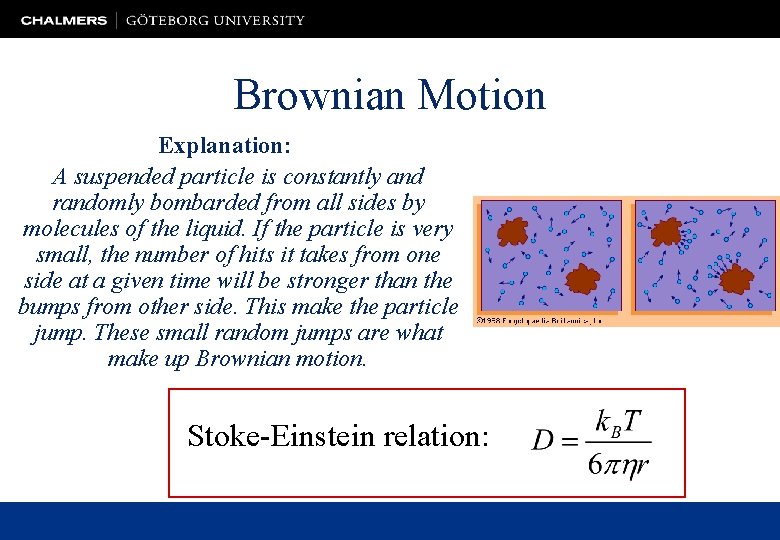
Brownian Motion • First observed in 1827 by the botanist Robert Brown. He was looking at pollen grains under a microscope. The force of life? • Desaulx in 1877: "In my way of thinking the phenomenon is a result of thermal molecular motion in the liquid environment (of the particles). ” • The mathematical theory of Brownian motion was developed by Einstein in 1905. • Jean-Baptiste Perrin verified Einstein's analysis (Nobel Prize 1926).

Brownian Motion Explanation: A suspended particle is constantly and randomly bombarded from all sides by molecules of the liquid. If the particle is very small, the number of hits it takes from one side at a given time will be stronger than the bumps from other side. This make the particle jump. These small random jumps are what make up Brownian motion. Stoke-Einstein relation:

Applications of DLS Size: Using Stoke-Einstein equation DLS can be used to easy, fast and accurate determination of the hydrodynamic radius of particles. Typically range: 1 nm – 1μm. Shape: Ellipsodial particles results in a small fraction depolarized scattered light. Can be used for estimation of ellipticity of the particles. Difficult!

Examples Some examples of sub-micron systems: – – – – Micro-emulsions peptides micelles macromolecules polymers paint pigments bacteria, viruses

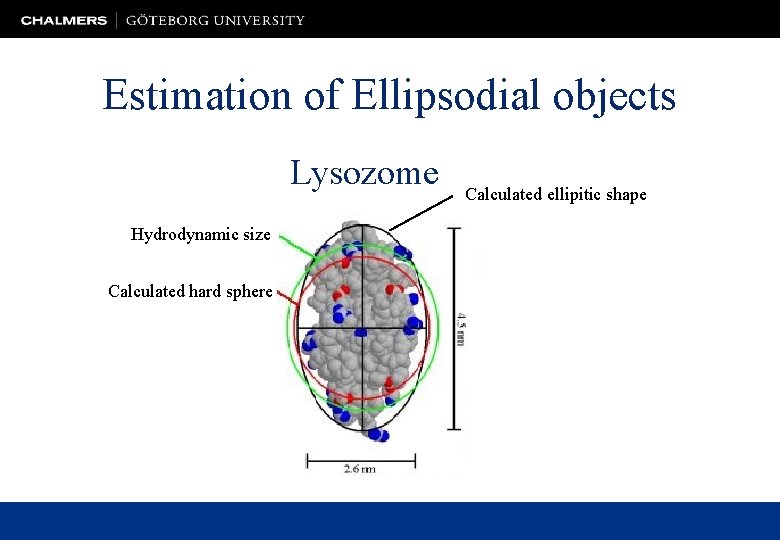
Estimation of Ellipsodial objects Lysozome Hydrodynamic size Calculated hard sphere Calculated ellipitic shape

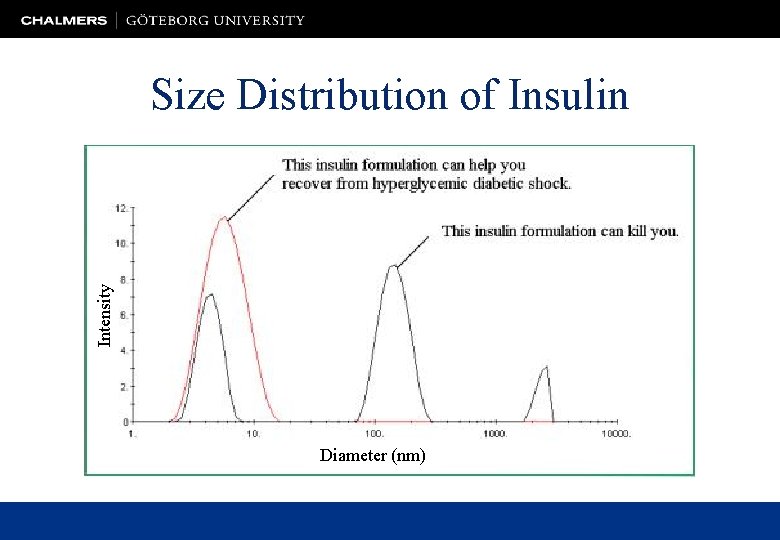
Intensity Size Distribution of Insulin Diameter (nm)

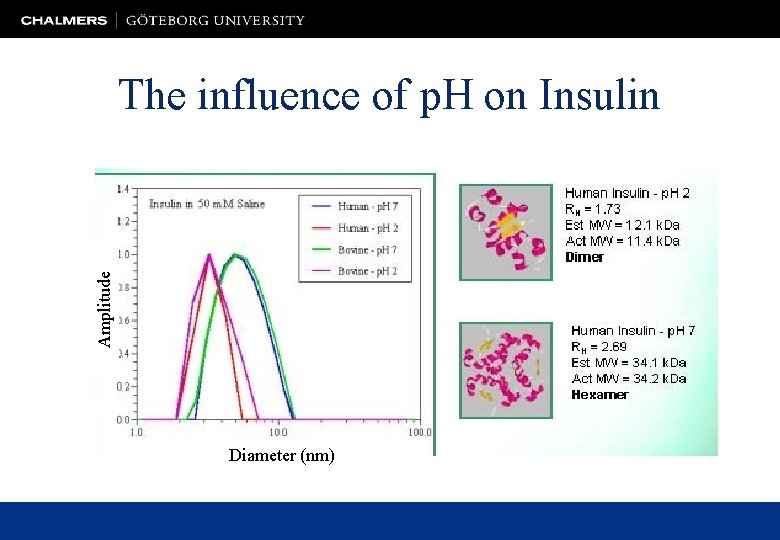
Amplitude The influence of p. H on Insulin Diameter (nm)

Research using DLS • Determination of size on complex systems: – “water-in-oil” – bio-molecules – cellulose • Glass transition dynamics • Polymer dynamics

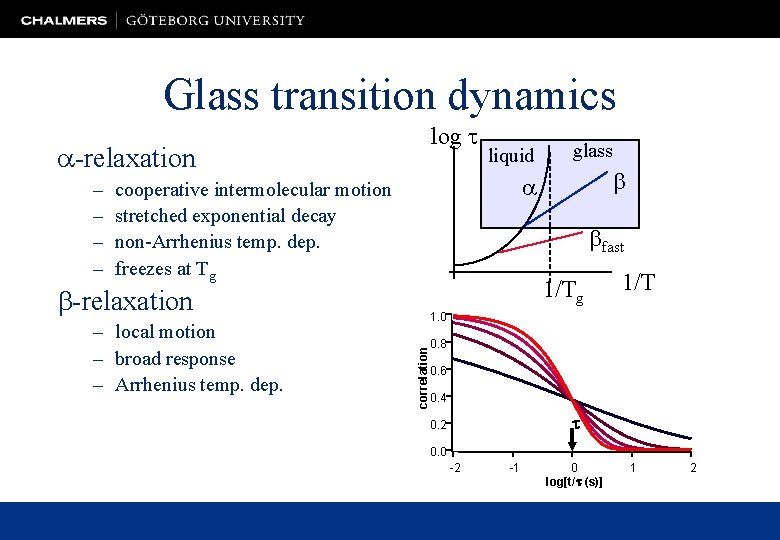
Glass transition dynamics -relaxation – – log liquid cooperative intermolecular motion stretched exponential decay non-Arrhenius temp. dep. freezes at Tg fast 1/Tg -relaxation 1/T 1. 0 correlation – local motion – broad response – Arrhenius temp. dep. glass 0. 8 0. 6 0. 4 t 0. 2 0. 0 -2 -1 0 log[t/t (s)] 1 2

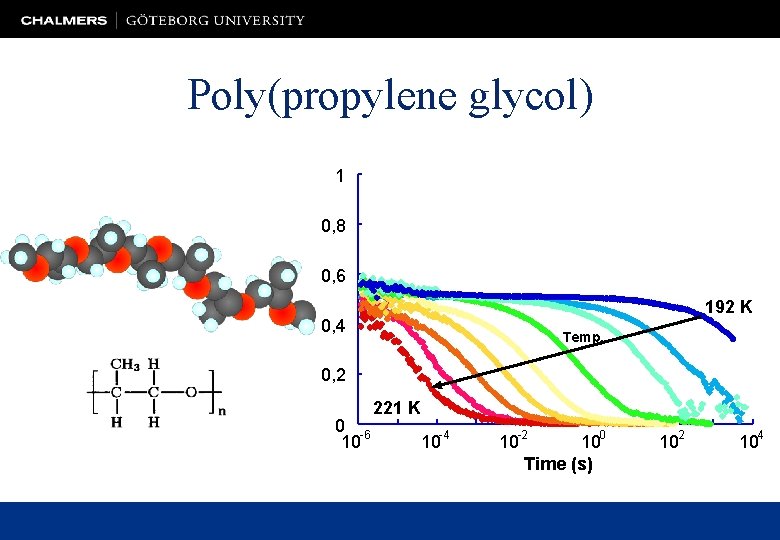
Poly(propylene glycol) 1 0, 8 0, 6 192 K 0, 4 Temp. 0, 2 0 -6 10 221 K 10 -4 10 -2 100 Time (s) 102 104
![Arrhenius Plot log Relaxation time s 4 Polymer n69 Dimer n2 0 Oligomer n7 Arrhenius Plot log[ Relaxation time (s)] 4 Polymer (n=69) Dimer (n=2) 0 Oligomer (n=7)](https://slidetodoc.com/presentation_image_h/7f248dbc6b9c1f3c54e3fc60097e89cc/image-21.jpg)
Arrhenius Plot log[ Relaxation time (s)] 4 Polymer (n=69) Dimer (n=2) 0 Oligomer (n=7) Conclusion: Shorter polymer chains relax faster than long chains. Monomer (n=1) -4 -8 -12 2 2. 5 3 3. 5 4 4. 5 1000/T (1/K) 5 5. 5 6

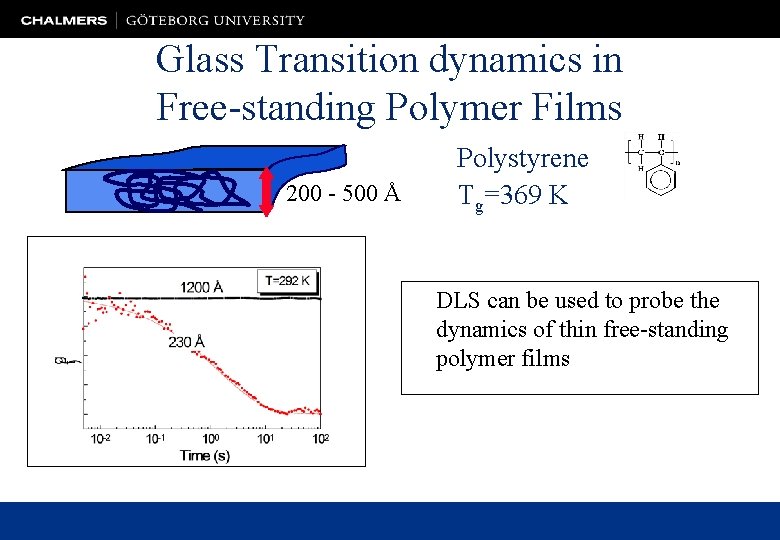
Glass Transition dynamics in Free-standing Polymer Films 200 - 500 Å Polystyrene Tg=369 K DLS can be used to probe the dynamics of thin free-standing polymer films

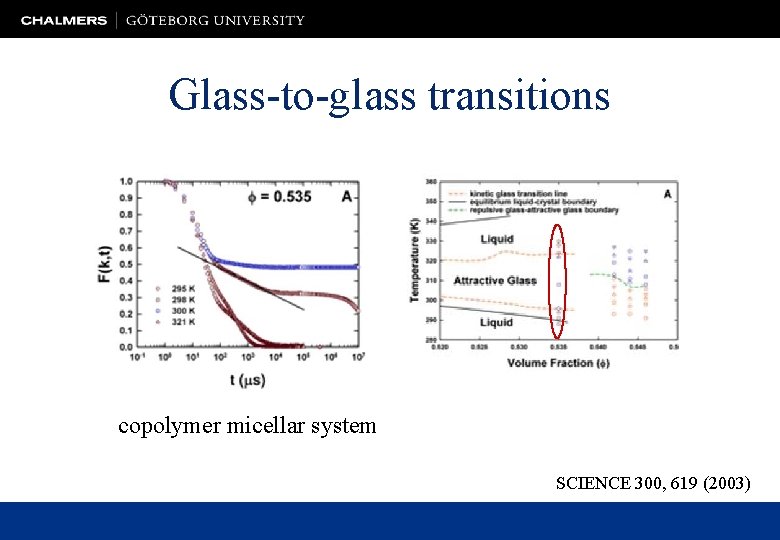
Glass-to-glass transitions copolymer micellar system SCIENCE 300, 619 (2003)

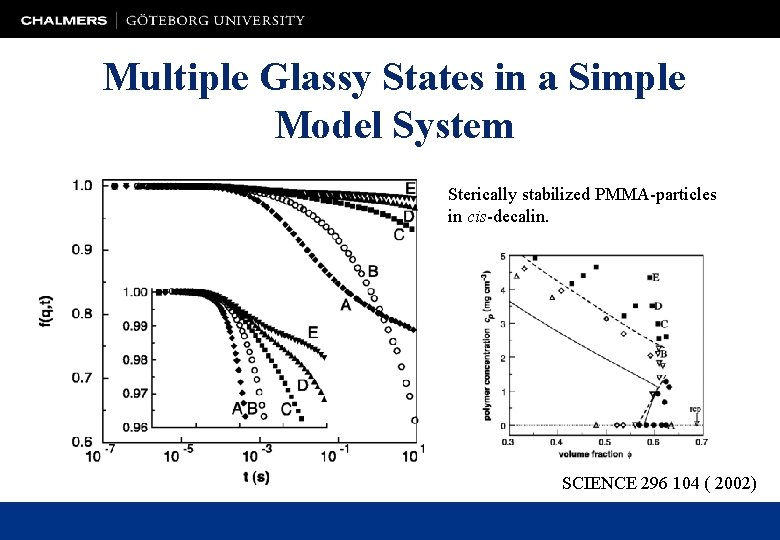
Multiple Glassy States in a Simple Model System Sterically stabilized PMMA-particles in cis-decalin. SCIENCE 296 104 ( 2002)

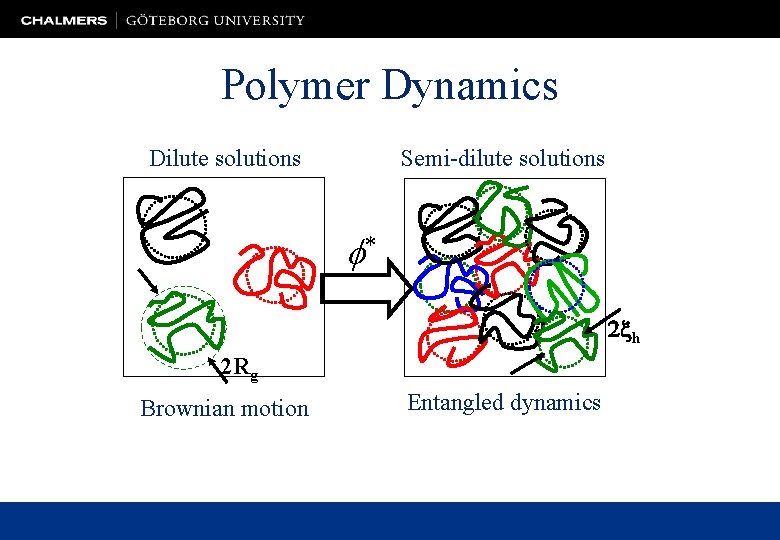
Polymer Dynamics Dilute solutions Semi-dilute solutions f* 2 xh 2 Rg Brownian motion Entangled dynamics

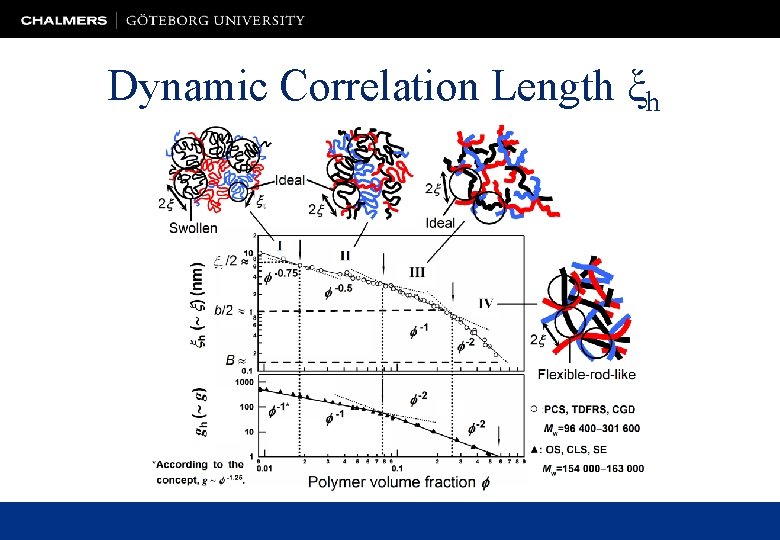
Dynamic Correlation Length ξh

Polymer Gel Electrolytes Poly(methyl methacrylate) + Propylene Carbonate / Ethylene Carbonate + Lithium Perchlorate (Li. Cl. O 4)

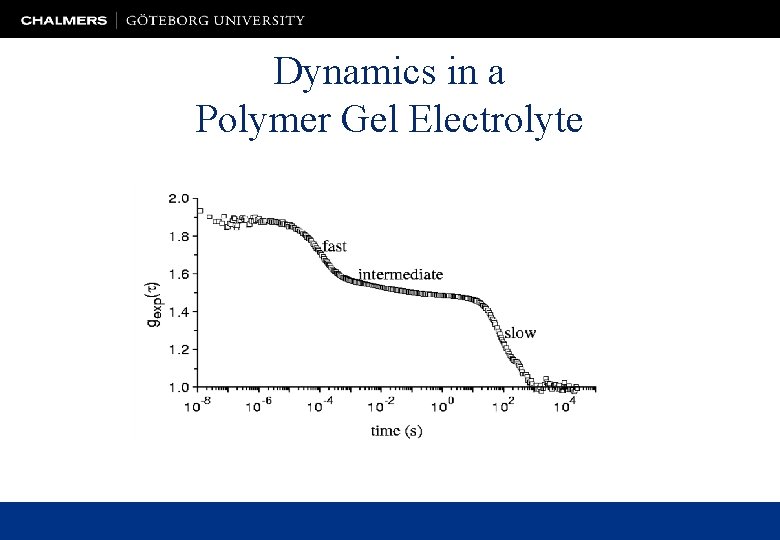
Dynamics in a Polymer Gel Electrolyte

Relaxations and Conductivity in Polymer Gel Electrolytes Nernst-Einstein equation …there is a close connection between the fast diffusive process and the ionic conductivity!

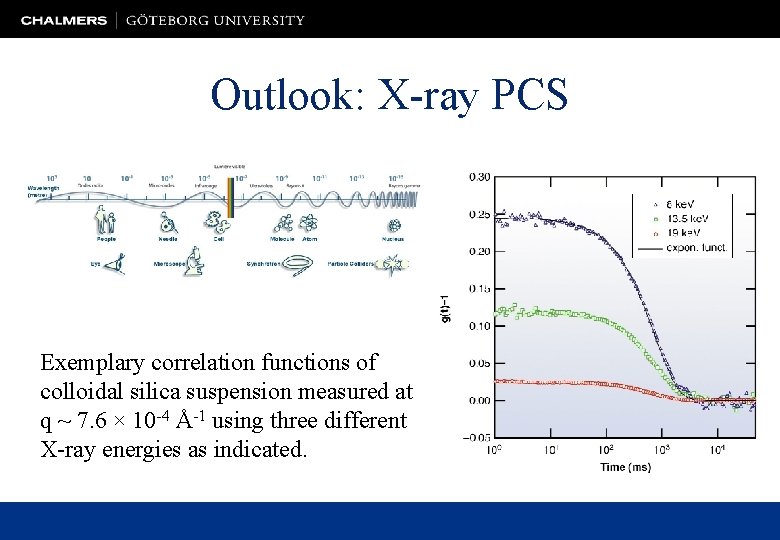
Outlook: X-ray PCS Exemplary correlation functions of colloidal silica suspension measured at q ~ 7. 6 × 10 -4 Å-1 using three different X-ray energies as indicated.

Summary: DLS technique Probes density and/or concentration fluctuations. Time scales: ~10 -6 - 103 s Wave vectors: ~10 -3 Å-1 Standard characterisation techniques for particles – Determination of size 1 nm - 1 mm – Estimation of ellipticity and/or swelling Research – Polymer dynamics – Glass transition dynamics