Intermolecular Forces IMF Section 6 5 p 203




- Slides: 9

Intermolecular Forces (IMF) Section 6. 5 p. 203 11/20/14

IMF General Info • The impact that intermolecular forces have on molecules can be seen on how boiling points are impacted. – Reference Table 7 p. 204 • Generally, these forces are weaker than actual chemical bonds, but are still important in examining the properties of molecules.

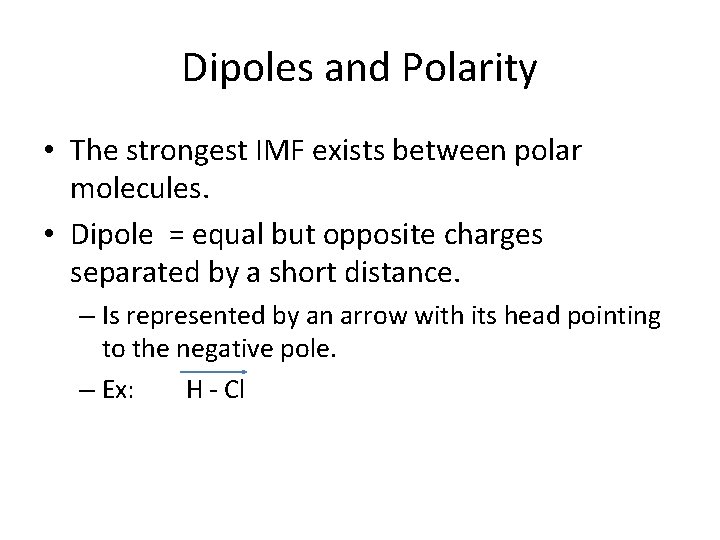
Dipoles and Polarity • The strongest IMF exists between polar molecules. • Dipole = equal but opposite charges separated by a short distance. – Is represented by an arrow with its head pointing to the negative pole. – Ex: H - Cl

Dipole and Polarity • Polarity of diatomic molecules is determined by 1 Bond. • Polarity for molecules with more than 1 Bond is determined by orientation of each bond (aka molecular geometry / shape). – Figure 26 p. 205 • Ex: CO 2 -> bond polarities between each C = O cancel each other out bc the extend equally and symmetrically in opposite directions.

London Dispersion Forces • Even Noble gas molecules that are nonpolar experience weak IMF attraction. • This is because electrons are in constant motion and may cause the electron distribution to be uneven within an atom / molecule. • When this happens instantaneous dipoles are formed = “London dispersion forces. ”

London Dispersion Forces (LDFs) • These forces increase with the number of electrons present. • So there is a trend: – LDFs increase with increasing atomic # – Ex: Low boiling point of Noble Gases.

Practice Problems IMF • Complete p. 210 -211 #33 -42, 43, 45, 46, 50

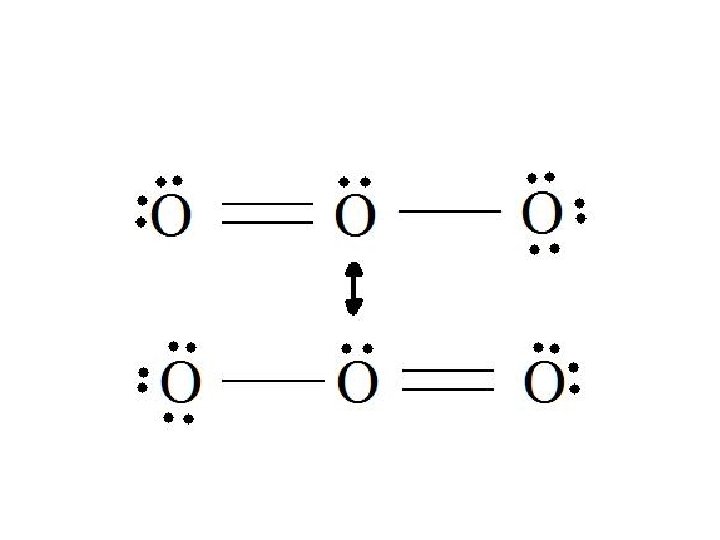
Resonance Structures • Some molecules cannot be represented adequately by a single Lewis Structure. • Ex: Ozone – O 3 • In this example the two structure of ozone are constantly alternating and therefore is no average structure but 2 plausible version. • We use a double headed arrow in between the Lewis Structures to represent this.
