Implanted Artificial Pancreas ACME Devices Company 1 ACME










- Slides: 21

Implanted Artificial Pancreas ACME Devices Company 1

ACME Devices Company: Implantable Artificial Pancreas Outline CMS Gap Analysis RWE Applications 2

Market Cap: $5. 7 Billion Employees: 15, 000 ACME Devices Company Global Headquarters: Chicago, IL Business Sector Activity • Diabetes Management • Cardiac Rhythm Management • Orthopedic Implants 3

Diabetes is prevalent and leads to heart disease, stroke and other serious disability Approximately 1. 6 million Americans with Type 1 Diabetes Intensive diabetes therapy has clear benefits • Reducing risk of hypo- and hyperglycemia • Reducing glycemic variability • Improving quality of life Existing approaches to intensive therapy • Require significant patient effort • Increase risk of hypoglycemia • May result in weight gain that undermines patient adherence • May cost 3 x more than conventional therapy The company, device, technologies, services, and clinical trials in this case study are hypothetical and are presented to facilitate a discussion of real world evidence development. The gap analysis is focused on major issues. This presentation in no way represents a finding by CMS that an applicable benefit category exists nor does it represent a commitment to a specific coverage determination should an implantable artificial pancreas be developed in the future.

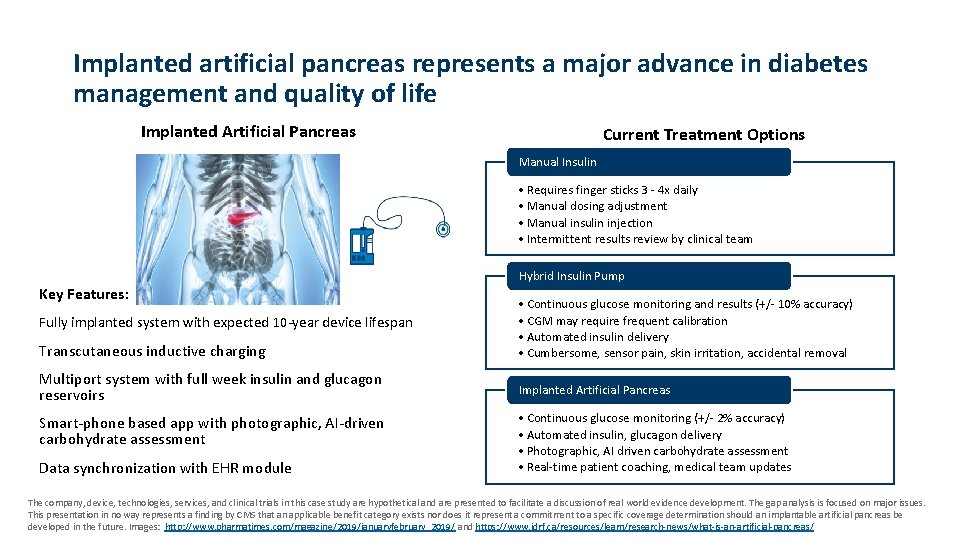
Implanted artificial pancreas represents a major advance in diabetes management and quality of life Implanted Artificial Pancreas Current Treatment Options Manual Insulin • Requires finger sticks 3 - 4 x daily • Manual dosing adjustment • Manual insulin injection • Intermittent results review by clinical team Hybrid Insulin Pump Key Features: Fully implanted system with expected 10 -year device lifespan Transcutaneous inductive charging Multiport system with full week insulin and glucagon reservoirs Smart-phone based app with photographic, AI-driven carbohydrate assessment Data synchronization with EHR module • Continuous glucose monitoring and results (+/- 10% accuracy) • CGM may require frequent calibration • Automated insulin delivery • Cumbersome, sensor pain, skin irritation, accidental removal Implanted Artificial Pancreas • Continuous glucose monitoring (+/- 2% accuracy) • Automated insulin, glucagon delivery • Photographic, AI driven carbohydrate assessment • Real-time patient coaching, medical team updates The company, device, technologies, services, and clinical trials in this case study are hypothetical and are presented to facilitate a discussion of real world evidence development. The gap analysis is focused on major issues. This presentation in no way represents a finding by CMS that an applicable benefit category exists nor does it represent a commitment to a specific coverage determination should an implantable artificial pancreas be developed in the future. Images: http: //www. pharmatimes. com/magazine/2019/januaryfebruary_2019/ and https: //www. jdrf. ca/resources/learn/research-news/what-is-an-artificial-pancreas/

Implanted artificial pancreas addresses lifestyle factors, improves care, and simplifies insulin management Simple, elegant system for diabetes management • Compatible with active lifestyles, convenient rapid charging and weekly reservoir refill • Smartphone dashboard: glucose values, battery, insulin / glucagon supply • AI-driven assessment of carbohydrates and calories, dietician coaching Addresses multiple risk factors and facilitates proactive care • Synchronizes with EHR data, including lipids, HA 1 C, weight, blood pressure • Automated patient / medical team alerts for aberrant values / trends, push notification for annual ophthalmology screening and flu shots The company, device, technologies, services, and clinical trials in this case study are hypothetical and are presented to facilitate a discussion of real world evidence development. The gap analysis is focused on major issues. This presentation in no way represents a finding by CMS that an applicable benefit category exists nor does it represent a commitment to a specific coverage determination should an implantable artificial pancreas be developed in the future.

Findings from the multicenter SWEET Relief trial Multi-center RCT with enrollment from US, Canada. 24 months of data. Two comparison groups: Self-administered insulin, Implanted Bihormonal Artificial Pancreas. Primary outcomes: • Serious hyperglycemic episodes requiring hospitalization • Serious hypoglycemic episodes (BS < 70) • Average glucose values • HA 1 C % values Secondary outcomes: • Surgical complications • Device failures requiring revision / extraction • Device or port infections The company, device, technologies, services, and clinical trials in this case study are hypothetical and are presented to facilitate a discussion of real world evidence development. The gap analysis is focused on major issues. This presentation in no way represents a finding by CMS that an applicable benefit category exists nor does it represent a commitment to a specific coverage determination should an implantable artificial pancreas be developed in the future.

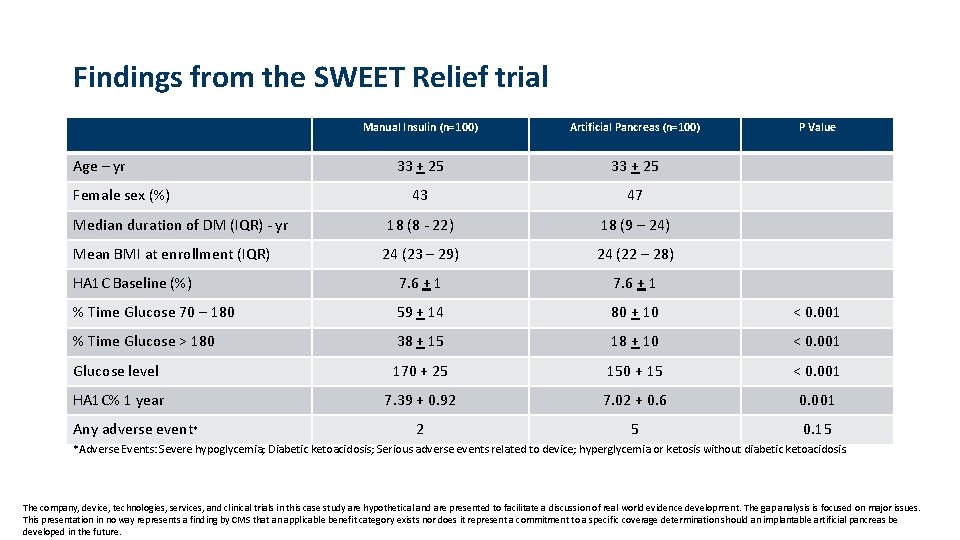
Findings from the SWEET Relief trial Manual Insulin (n=100) Artificial Pancreas (n=100) 33 + 25 43 47 18 (8 - 22) 18 (9 – 24) 24 (23 – 29) 24 (22 – 28) HA 1 C Baseline (%) 7. 6 + 1 % Time Glucose 70 – 180 59 + 14 80 + 10 < 0. 001 % Time Glucose > 180 38 + 15 18 + 10 < 0. 001 Glucose level 170 + 25 150 + 15 < 0. 001 HA 1 C% 1 year 7. 39 + 0. 92 7. 02 + 0. 6 0. 001 2 5 0. 15 Age – yr Female sex (%) Median duration of DM (IQR) - yr Mean BMI at enrollment (IQR) Any adverse event* P Value *Adverse Events: Severe hypoglycemia; Diabetic ketoacidosis; Serious adverse events related to device; hyperglycemia or ketosis without diabetic ketoacidosis. The company, device, technologies, services, and clinical trials in this case study are hypothetical and are presented to facilitate a discussion of real world evidence development. The gap analysis is focused on major issues. This presentation in no way represents a finding by CMS that an applicable benefit category exists nor does it represent a commitment to a specific coverage determination should an implantable artificial pancreas be developed in the future.

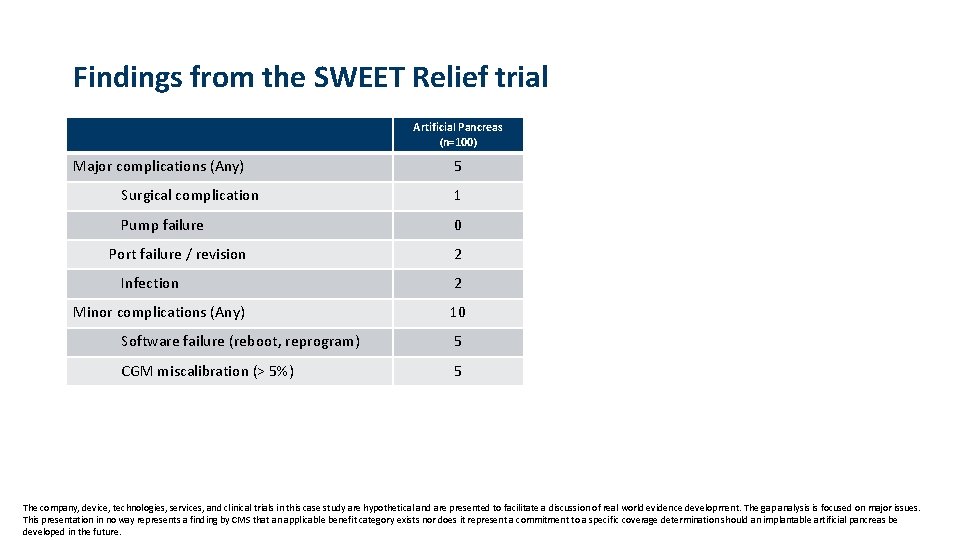
Findings from the SWEET Relief trial Artificial Pancreas (n=100) Major complications (Any) 5 Surgical complication 1 Pump failure 0 Port failure / revision Infection Minor complications (Any) 2 2 10 Software failure (reboot, reprogram) 5 CGM miscalibration (> 5%) 5 The company, device, technologies, services, and clinical trials in this case study are hypothetical and are presented to facilitate a discussion of real world evidence development. The gap analysis is focused on major issues. This presentation in no way represents a finding by CMS that an applicable benefit category exists nor does it represent a commitment to a specific coverage determination should an implantable artificial pancreas be developed in the future.

The CRUSH Diabetes trial Multi-center RCT with enrollment from US, Canada, and Germany. Two comparison groups: Self-administered insulin, Implanted Bihormonal Artificial Pancreas. Primary outcomes: • % time glucose in range 70 – 180 • % time glucose > 180 • HBA 1 C at 1 year Secondary outcomes: • Composite adverse event rate The company, device, technologies, services, and clinical trials in this case study are hypothetical and are presented to facilitate a discussion of real world evidence development. The gap analysis is focused on major issues. This presentation in no way represents a finding by CMS that an applicable benefit category exists nor does it represent a commitment to a specific coverage determination should an implantable artificial pancreas be developed in the future.

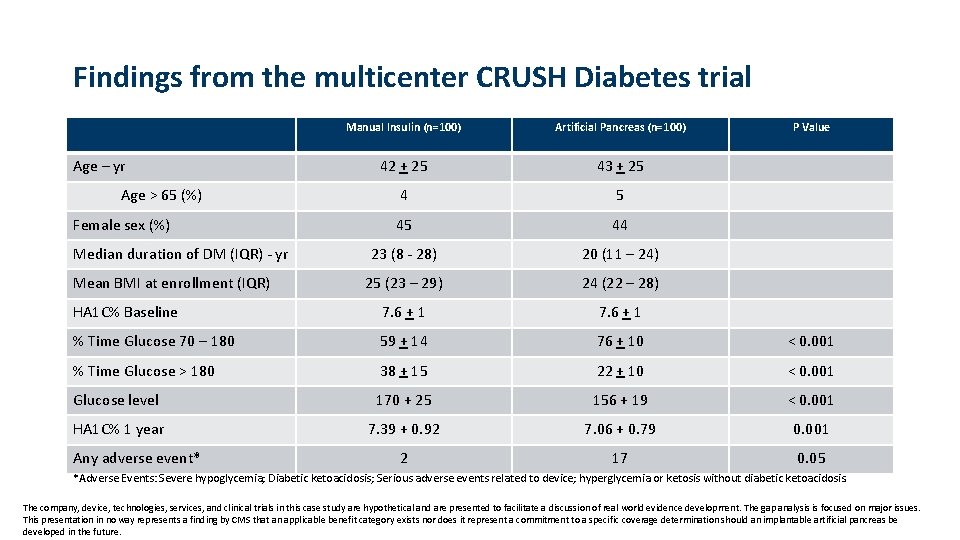
Findings from the multicenter CRUSH Diabetes trial Manual Insulin (n=100) Artificial Pancreas (n=100) 42 + 25 43 + 25 4 5 45 44 23 (8 - 28) 20 (11 – 24) 25 (23 – 29) 24 (22 – 28) HA 1 C% Baseline 7. 6 + 1 % Time Glucose 70 – 180 59 + 14 76 + 10 < 0. 001 % Time Glucose > 180 38 + 15 22 + 10 < 0. 001 Glucose level 170 + 25 156 + 19 < 0. 001 HA 1 C% 1 year 7. 39 + 0. 92 7. 06 + 0. 79 0. 001 2 17 0. 05 Age – yr Age > 65 (%) Female sex (%) Median duration of DM (IQR) - yr Mean BMI at enrollment (IQR) Any adverse event* P Value *Adverse Events: Severe hypoglycemia; Diabetic ketoacidosis; Serious adverse events related to device; hyperglycemia or ketosis without diabetic ketoacidosis. The company, device, technologies, services, and clinical trials in this case study are hypothetical and are presented to facilitate a discussion of real world evidence development. The gap analysis is focused on major issues. This presentation in no way represents a finding by CMS that an applicable benefit category exists nor does it represent a commitment to a specific coverage determination should an implantable artificial pancreas be developed in the future.

FDA Labelling The Artificial Pancreas is intended for continuous delivery of basal and bolus insulin for the management of diabetes mellitus in persons, sixteen years of age and older, requiring insulin as well as for the continuous monitoring and trending of glucose levels in the fluid under the skin. The Artificial Pancreas can be programmed to automatically suspend insulin and deliver glucagon when the sensor glucose value falls below a predefined threshold value. The Artificial Pancreas System consists of the following devices that can be used individually or in combination: • Artificial Pancreas • Subcutaneous inductive charger • Insulin Port Plus • Next. Gen Diabetes software suite The company, device, technologies, services, and clinical trials in this case study are hypothetical and are presented to facilitate a discussion of real world evidence development. The gap analysis is focused on major issues. This presentation in no way represents a finding by CMS that an applicable benefit category exists nor does it represent a commitment to a specific coverage determination should an implantable artificial pancreas be developed in the future.

ACME Devices Company: Implantable Artificial Pancreas Outline CMS Gap Analysis RWE Applications 13

CMS Considerations • Is there an applicable Benefit Category? • After thorough review of the available evidence, does the item or service meet the “reasonable and necessary” definition? • If the item or service is promising but does not yet meet the “reasonable and necessary” definition, what evidence gaps remain? The company, device, technologies, services, and clinical trials in this case study are hypothetical and are presented to facilitate a discussion of real world evidence development. The gap analysis is focused on major issues. This presentation in no way represents a finding by CMS that an applicable benefit category exists nor does it represent a commitment to a specific coverage determination should an implantable artificial pancreas be developed in the future.

Reasonable and Necessary Definition Reasonable and necessary means an item or service is (1) safe and effective, (2) not experimental or investigational, (3) appropriate for Medicare patients Appropriate for Medicare Patients, including the duration and frequency that is considered appropriate for the item or service, in terms of whether it is: • Furnished in accordance with accepted standards of medical practice for the diagnosis or treatment of the patient's condition or to improve the function of a malformed body member • Furnished in a setting appropriate to the patient's medical needs and condition • Ordered and furnished by qualified personnel • One that meets, but does not exceed, the patient's medical need • At least as beneficial as an existing and available medically appropriate alternative The company, device, technologies, services, and clinical trials in this case study are hypothetical and are presented to facilitate a discussion of real world evidence development. The gap analysis is focused on major issues. This presentation in no way represents a finding by CMS that an applicable benefit category exists nor does it represent a commitment to a specific coverage determination should an implantable artificial pancreas be developed in the future.

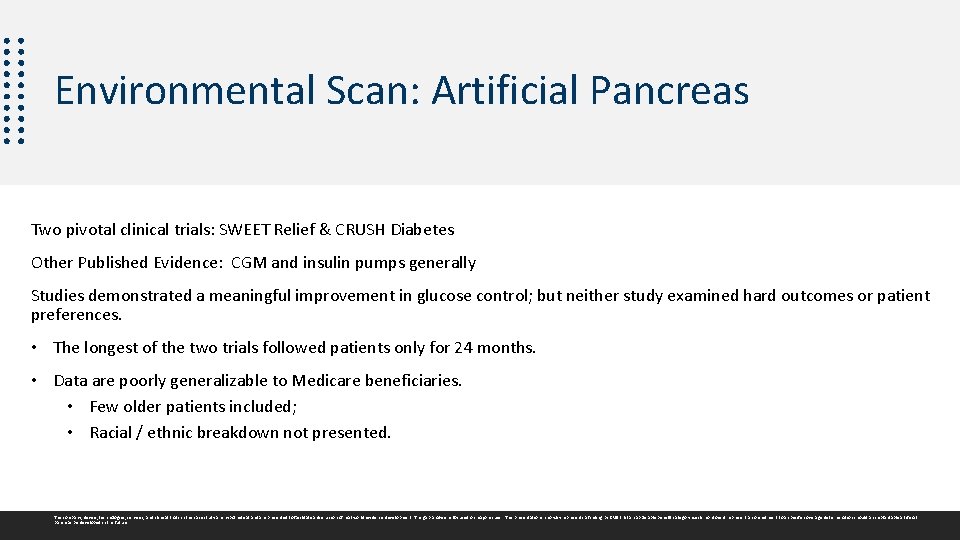
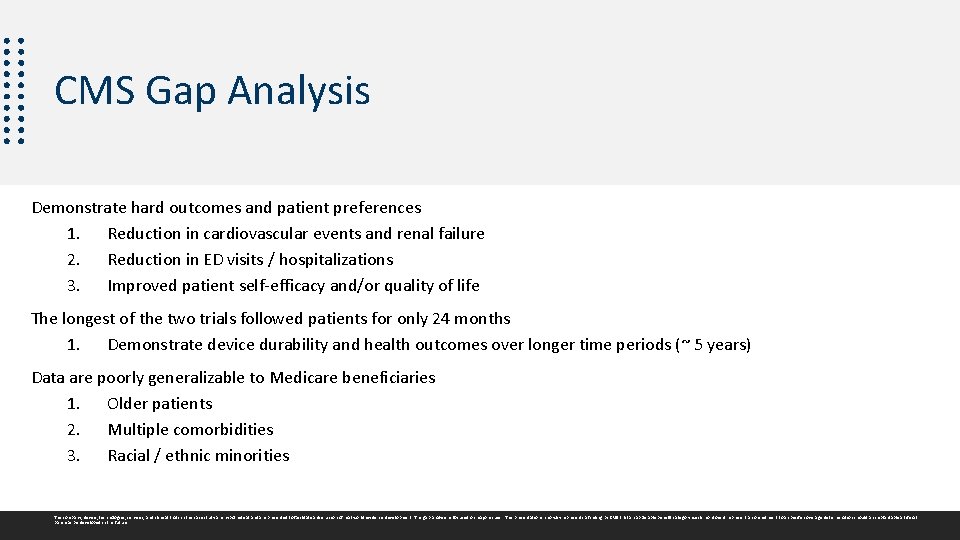
Environmental Scan: Artificial Pancreas Two pivotal clinical trials: SWEET Relief & CRUSH Diabetes Other Published Evidence: CGM and insulin pumps generally Studies demonstrated a meaningful improvement in glucose control; but neither study examined hard outcomes or patient preferences. • The longest of the two trials followed patients only for 24 months. • Data are poorly generalizable to Medicare beneficiaries. • Few older patients included; • Racial / ethnic breakdown not presented. The company, device, technologies, services, and clinical trials in this case study are hypothetical and are presented to facilitate a discussion of real world evidence development. The gap analysis is focused on major issues. This presentation in no way represents a finding by CMS that an applicable benefit category exists nor does it represent a commitment to a specific coverage determination should an implantable artificial pancreas be developed in the future.

CMS Gap Analysis Demonstrate hard outcomes and patient preferences 1. Reduction in cardiovascular events and renal failure 2. Reduction in ED visits / hospitalizations 3. Improved patient self-efficacy and/or quality of life The longest of the two trials followed patients for only 24 months 1. Demonstrate device durability and health outcomes over longer time periods (~ 5 years) Data are poorly generalizable to Medicare beneficiaries 1. Older patients 2. Multiple comorbidities 3. Racial / ethnic minorities The company, device, technologies, services, and clinical trials in this case study are hypothetical and are presented to facilitate a discussion of real world evidence development. The gap analysis is focused on major issues. This presentation in no way represents a finding by CMS that an applicable benefit category exists nor does it represent a commitment to a specific coverage determination should an implantable artificial pancreas be developed in the future.

ACME Devices Company: Implantable Artificial Pancreas Outline CMS Gap Analysis RWE Applications 18

How might RWE be used to “close the gap”? 19

The company, device, technologies, services, and clinical trials n this case study are hypothetical and are presented to facilitate a discussion of real world evidence development. The gap analysis is focused on major issues. This presentation in no way represents a finding by CMS that an applicable benefit category exists nor does it represent a commitment to a specific coverage determination should an implantable artificial pancreas be developed in the future.

The company, device, technologies, services, and clinical trials n this case study are hypothetical and are presented to facilitate a discussion of real world evidence development. The gap analysis is focused on major issues. This presentation in no way represents a finding by CMS that an applicable benefit category exists nor does it represent a commitment to a specific coverage determination should an implantable artificial pancreas be developed in the future.
 The implanted word
The implanted word Acme manufacturing company
Acme manufacturing company Acme portable corp
Acme portable corp Suppose the acme drug company develops
Suppose the acme drug company develops Acme manufacturing company case study
Acme manufacturing company case study Acme toy company prints baseball cards
Acme toy company prints baseball cards Artificial intelligence devices
Artificial intelligence devices Artificial intelligence devices
Artificial intelligence devices Artificial intelligence devices
Artificial intelligence devices Electronic device designed to accept data
Electronic device designed to accept data Artificial intelligence devices
Artificial intelligence devices Acme planimeter
Acme planimeter Acme stamping value stream mapping
Acme stamping value stream mapping Acme construction
Acme construction Acme workflow management
Acme workflow management Acme database
Acme database Acme corporation strategic plan
Acme corporation strategic plan Pulso celer
Pulso celer Paratech rescue team
Paratech rescue team Acme bolt trucking game
Acme bolt trucking game Acme corporation strategic plan
Acme corporation strategic plan Acme omega
Acme omega