If youre here for Chem 1114 youre in








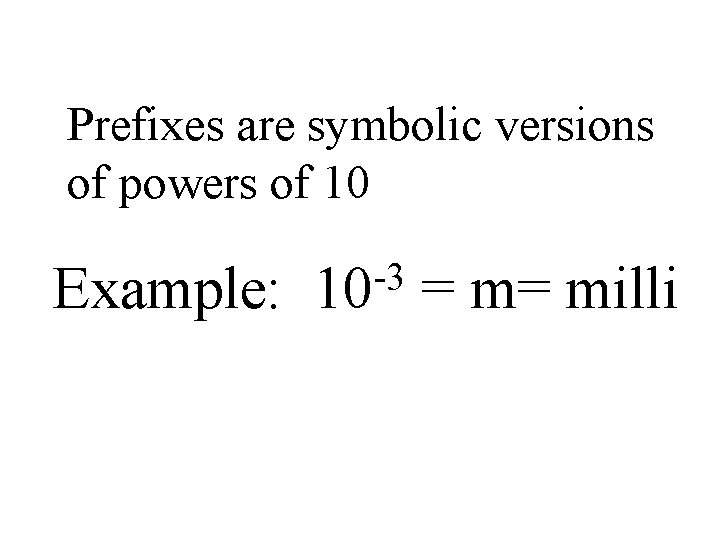
- Slides: 16

If you’re here for Chem 1114, you’re in the right place.

CHEM 1114: BASIC STUFF YOU NEED • Course Website (see also: Course Syllabus) https: //web. alfredstate. edu/fongjd/Gen. Chem 1. htm • Course Syllabus (See also: Course Website) • Zumdahl Hybrid E-text • Scientific Calculator

YOUR FIRST ASSIGNMENT By Wednesday 2 Sept: • Read Course Syllabus (handout in class) • Read Fong CV (curriculum vitae) and `A letter from your chemistry 1114 instructor’ Both found under Miscellaneous at course website: https: //web. alfredstate. edu/fongjd/Gen. Chem 1. htm • Write me a letter (no longer than 1 page) about yourself and turn the hard copy in during class (do not e-mail me!). (3 pts) • Start reading text (Zumdahl & Zumdahl 9 th pp. 8 -22)

WHY CHEMISTRY ? Your thoughts ?

Can you locate the chemist’s universal obsession…. . ? Structure Chemist Organic Chemist Nut case chemist

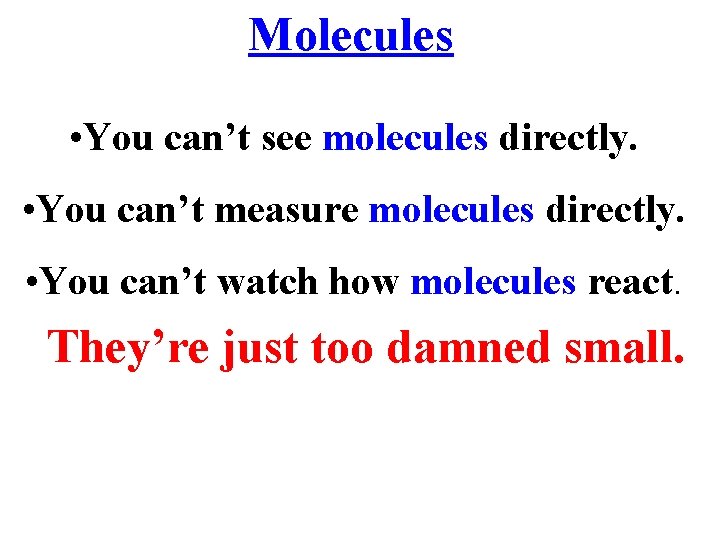
Molecules • You can’t see molecules directly. • You can’t measure molecules directly. • You can’t watch how molecules react. They’re just too damned small.

Chemistry involves imaginative, BUT deeply abstract metaphors for things (molecules) that we can’t know directly.

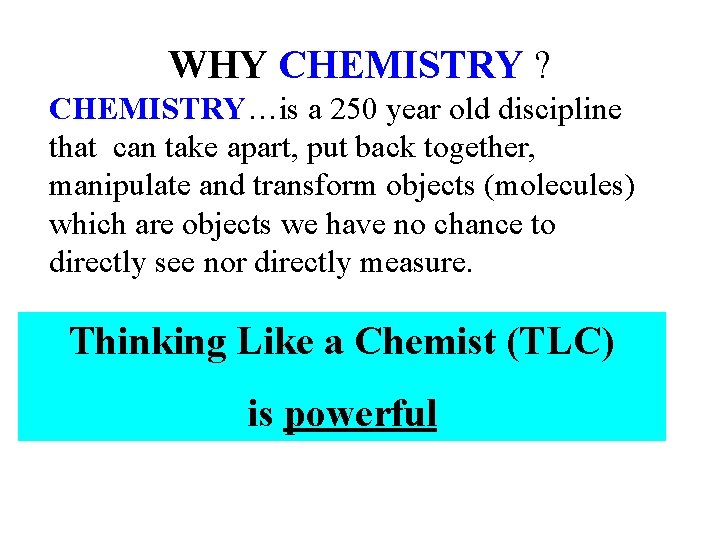
WHY CHEMISTRY ? CHEMISTRY…is a 250 year old discipline that can take apart, put back together, manipulate and transform objects (molecules) which are objects we have no chance to directly see nor directly measure. Thinking Like a Chemist (TLC) is powerful

Where do we start to get TLC powerful ? From COURSE OUTLINE Week dates 1 8/31 -9/4 lecture topics reading Units, Prefixes, Sig Figs and pp. 8 -22 Measurement Concepts: the ground floor

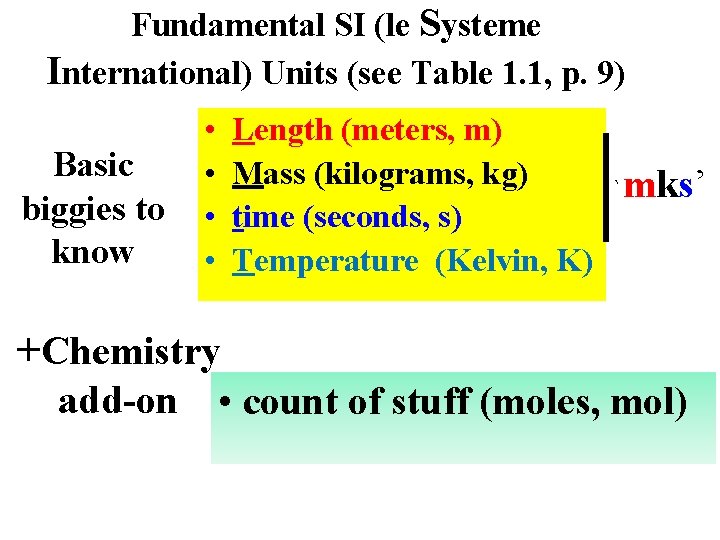
Fundamental SI (le Systeme International) Units (see Table 1. 1, p. 9) Basic biggies to know • • Length (meters, m) Mass (kilograms, kg) time (seconds, s) Temperature (Kelvin, K) `mks’ +Chemistry add-on • count of stuff (moles, mol)

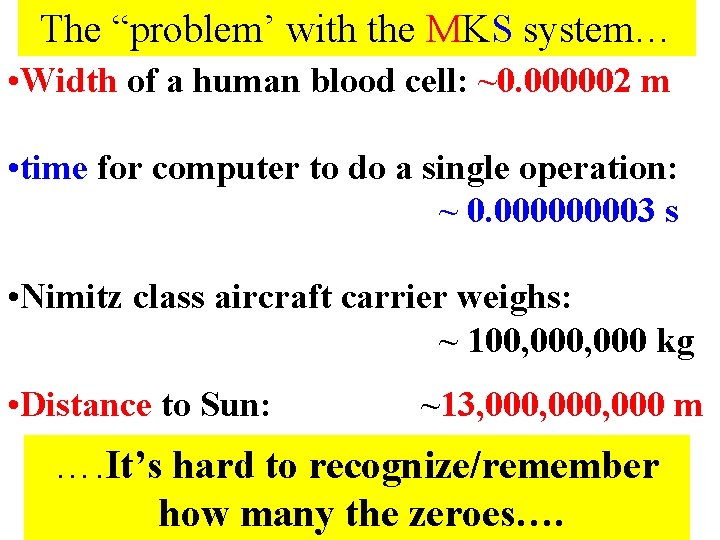
The “problem’ with the MKS system… • Width of a human blood cell: ~0. 000002 m • time for computer to do a single operation: ~ 0. 00003 s • Nimitz class aircraft carrier weighs: ~ 100, 000 kg • Distance to Sun: ~13, 000, 000 m …. It’s hard to recognize/remember how many the zeroes….

Two ways to keep track of the zeroes and the decimal place: • scientific notation • Prefixes (see table 1. 2, p. 10)

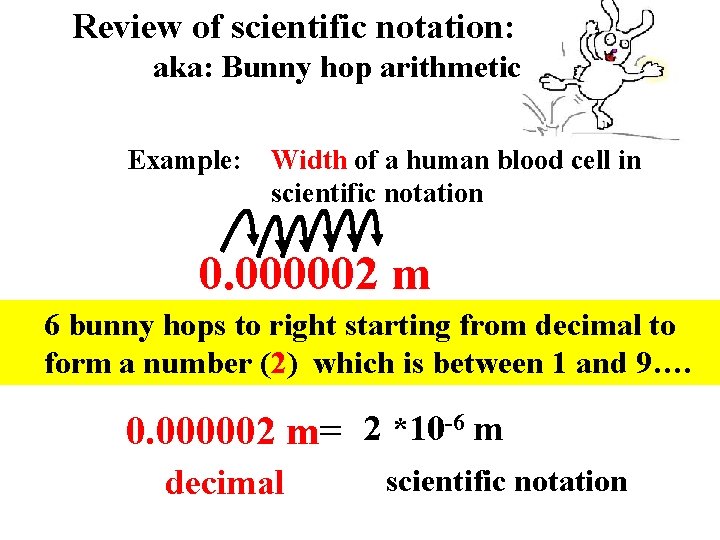
Review of scientific notation: aka: Bunny hop arithmetic Example: Width of a human blood cell in scientific notation 0. 000002 m 6 bunny hops to right starting from decimal to form a number (2) which is between 1 and 9…. -6 m 2 *10 0. 000002 m= scientific notation decimal

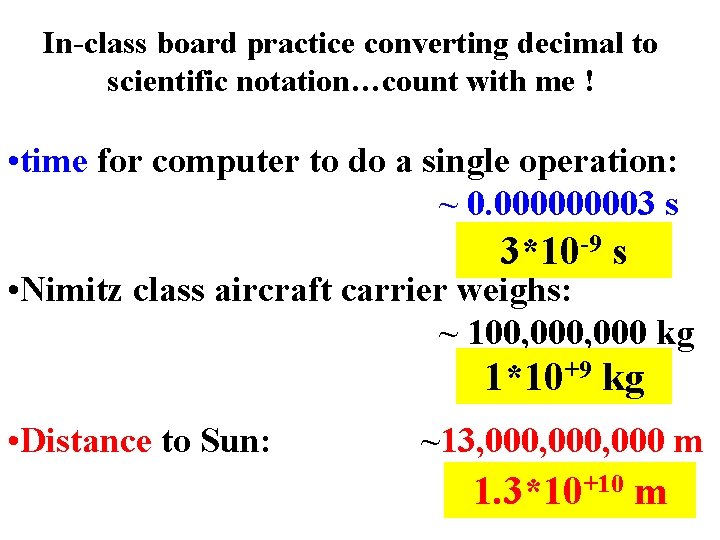
In-class board practice converting decimal to scientific notation…count with me ! • time for computer to do a single operation: ~ 0. 00003 s 3*10 -9 s • Nimitz class aircraft carrier weighs: ~ 100, 000 kg 1*10+9 kg • Distance to Sun: ~13, 000, 000 m 1. 3*10+10 m
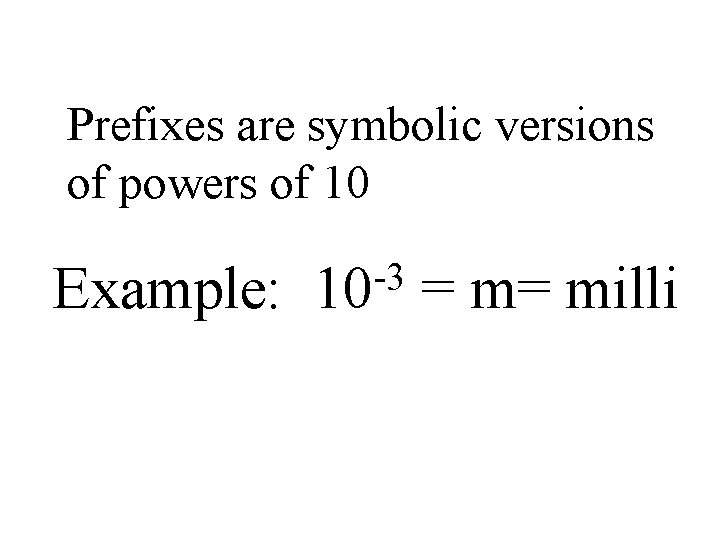
Prefixes are symbolic versions of powers of 10 Example: -3 10 = m= milli

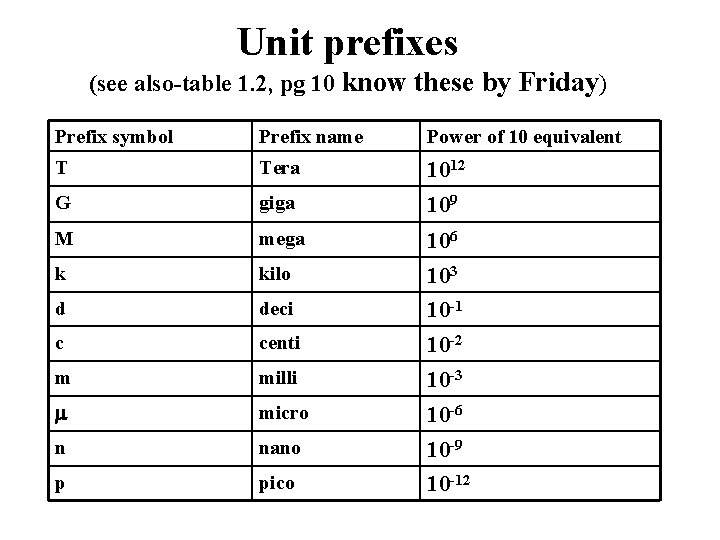
Unit prefixes (see also-table 1. 2, pg 10 know these by Friday) Prefix symbol Prefix name Power of 10 equivalent T Tera 1012 G giga 109 M mega 106 k kilo 103 d deci 10 -1 c centi 10 -2 m milli 10 -3 micro 10 -6 n nano 10 -9 p pico 10 -12
 Cs 1114
Cs 1114 There's a place where streams of grace
There's a place where streams of grace I dont know where youre going
I dont know where youre going Tentative goal statement
Tentative goal statement Jesus you are my firm foundation
Jesus you are my firm foundation Asexual symptoms
Asexual symptoms Professional letters
Professional letters Use the labels to draw and annotate a cell membrane
Use the labels to draw and annotate a cell membrane You're so random
You're so random Diamante poem about love
Diamante poem about love What to do when youre angry
What to do when youre angry Im ok youre not ok
Im ok youre not ok You're my master
You're my master How youre
How youre Hello everyone good morning
Hello everyone good morning Tack för att ni lyssnade
Tack för att ni lyssnade Att skriva debattartikel
Att skriva debattartikel