Huntington Disease in the 21 st Century Moderator



![Cognitive Symptoms • Subcortical cognitive impairment[a] – Insidious decline in 21 measures[b] – Dysfunction Cognitive Symptoms • Subcortical cognitive impairment[a] – Insidious decline in 21 measures[b] – Dysfunction](https://slidetodoc.com/presentation_image/0dc534486e4b8b9975facdbea2f0055e/image-7.jpg)
![Psychiatric and Behavioral Symptoms • Often earliest symptoms[a] – Depression, apathy, and other psychiatric Psychiatric and Behavioral Symptoms • Often earliest symptoms[a] – Depression, apathy, and other psychiatric](https://slidetodoc.com/presentation_image/0dc534486e4b8b9975facdbea2f0055e/image-8.jpg)


![Treating Cognitive Symptoms • Neural compensation seen in preclinical stages[a] • No accepted cognitive Treating Cognitive Symptoms • Neural compensation seen in preclinical stages[a] • No accepted cognitive](https://slidetodoc.com/presentation_image/0dc534486e4b8b9975facdbea2f0055e/image-15.jpg)
![Treating Chorea • Treat chorea to – Improve quality of life[a, b] – Prevent Treating Chorea • Treat chorea to – Improve quality of life[a, b] – Prevent](https://slidetodoc.com/presentation_image/0dc534486e4b8b9975facdbea2f0055e/image-17.jpg)
![Pharmacologic Treatment of Chorea Associated With HD • FDA-approved drugs – Tetrabenazine[a] and deutetrabenazine[b] Pharmacologic Treatment of Chorea Associated With HD • FDA-approved drugs – Tetrabenazine[a] and deutetrabenazine[b]](https://slidetodoc.com/presentation_image/0dc534486e4b8b9975facdbea2f0055e/image-18.jpg)
![Tetrabenazine and Deutetrabenazine Parameter Approval Pharmacokinetics[a] Tetrabenazine Deutetrabenazine 2008 2017 P Cmax, ng/m. L Tetrabenazine and Deutetrabenazine Parameter Approval Pharmacokinetics[a] Tetrabenazine Deutetrabenazine 2008 2017 P Cmax, ng/m. L](https://slidetodoc.com/presentation_image/0dc534486e4b8b9975facdbea2f0055e/image-19.jpg)



- Slides: 24

Huntington Disease in the 21 st Century Moderator Victor Sung, MD Associate Professor of Neurology Director, HDSA Center of Excellence University of Alabama at Birmingham, Alabama Panelist Erin Furr-Stimming, MD Associate Professor of Neurology Director, HDSA Center of Excellence University of Texas Health Science Center in Houston, Texas

This program will include a discussion of off-label treatment and investigational agents not approved by the FDA for use in the United States.

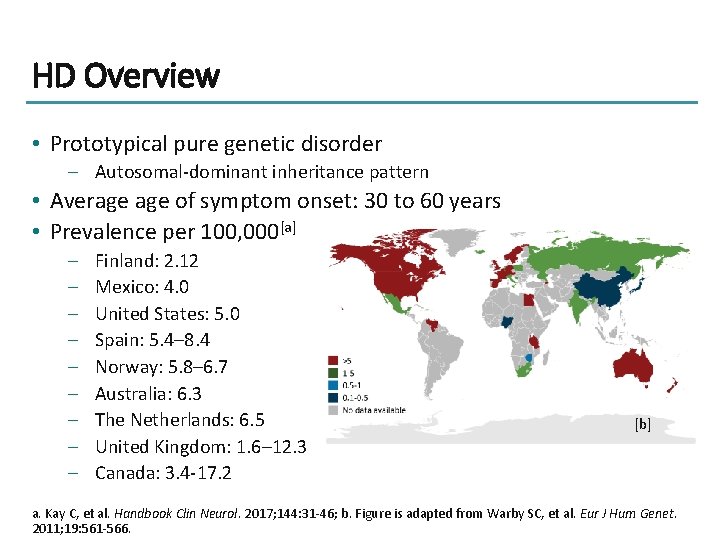
HD Overview • Prototypical pure genetic disorder – Autosomal-dominant inheritance pattern • Average of symptom onset: 30 to 60 years • Prevalence per 100, 000[a] – – – – – Finland: 2. 12 Mexico: 4. 0 United States: 5. 0 Spain: 5. 4– 8. 4 Norway: 5. 8– 6. 7 Australia: 6. 3 The Netherlands: 6. 5 United Kingdom: 1. 6– 12. 3 Canada: 3. 4 -17. 2 [b] a. Kay C, et al. Handbook Clin Neurol. 2017; 144: 31 -46; b. Figure is adapted from Warby SC, et al. Eur J Hum Genet. 2011; 19: 561 -566.

Genetic Basis Length of expanded CAG repeat • Varies between individuals because of its instability through intergenerational transmission • Is the primary determining factor of biologic changes resulting in HD • Inversely correlates with age of onset of: – Diagnostic motor signs – Cognitive symptoms • Is less correlated with onset of psychiatric symptoms • 40 to 55 CAG repeats typically associated with adult onset of motor symptoms Gusella JF, et al. Mov Disord. 2014; 29: 1359 -1365.

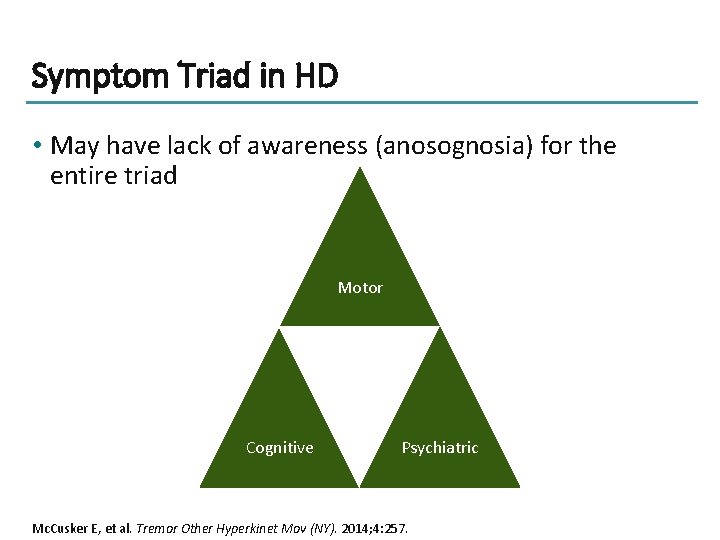
Symptom Triad in HD • May have lack of awareness (anosognosia) for the entire triad Motor Cognitive Psychiatric Mc. Cusker E, et al. Tremor Other Hyperkinet Mov (NY). 2014; 4: 257.

Motor Symptoms • Chorea – Abnormal, involuntary, purposeless, relatively random and quick movements – Affects gait, coordination, speech, and swallowing • Impairment of voluntary movements – Impersistence – Incoordination – Difficulty with fine motor skills • Less common – – – Bradykinesia Dystonia Myoclonus Slowing saccades Tics Jankovic J, et al. Mov Disord. 2014; 29: 1414 -1418; Nance M, et al. A Physicians’ Guide to the Management of Huntington’s Disease. ; 2011.
![Cognitive Symptoms Subcortical cognitive impairmenta Insidious decline in 21 measuresb Dysfunction Cognitive Symptoms • Subcortical cognitive impairment[a] – Insidious decline in 21 measures[b] – Dysfunction](https://slidetodoc.com/presentation_image/0dc534486e4b8b9975facdbea2f0055e/image-7.jpg)
Cognitive Symptoms • Subcortical cognitive impairment[a] – Insidious decline in 21 measures[b] – Dysfunction in attention, working memory, verbal learning, long-term memory, and learning of random associations may be earliest manifestations[b] – Memory relatively preserved[c] • Evident prior to motor dysfunction[b] • UHDRS TFC associated with cognitive performance[b] • SDMT can be used to track progression and detect onset of cognitive problems[c] • Cognitive reserve associated with slower rate of change in TMT-B and brain volume loss in prodromal HD[d] a. Papoutsi M, et al. Mov Disord. 2014; 29: 673 -683; b. Paulsen JS, et al. Mov Disord. 2014; 29: 1342 -1350; c. Lemiere J, et al. J Neurol. 2004; 251: 935 -942; d. Bonner-Jackson A, et al. J Int Neuropsychol Soc. 2014; 19: 739 -750.
![Psychiatric and Behavioral Symptoms Often earliest symptomsa Depression apathy and other psychiatric Psychiatric and Behavioral Symptoms • Often earliest symptoms[a] – Depression, apathy, and other psychiatric](https://slidetodoc.com/presentation_image/0dc534486e4b8b9975facdbea2f0055e/image-8.jpg)
Psychiatric and Behavioral Symptoms • Often earliest symptoms[a] – Depression, apathy, and other psychiatric symptoms as prodromal symptoms • Often most disruptive (though treatable) • Can include impulsivity, [b] depression, anxiety, hallucinations, delusion, perseveration, irritability, apathy, and personality change[c] • Combination of untreated depression and impulsivity can result in increased suicide risk[c] a. Martinez-Horta S, et al. Parkinsonism Relat Disord. 2016; 25: 58 -64; b. Johnson PL, et al. J Clin Exp Neuropsychol. 2017; 39: 694 -706; c. Cardoso F. Int Rev Neurobiol. 2017; 134: 1397 -1408.

HD Diagnosis • Based upon – Family history – Clinical presentation with typical motor, behavioral, or cognitive symptoms • Confirmed with definitive genetic testing • Supportive findings on neuroimaging – The presence of caudate atrophy is but not diagnostic in and of itself Pagan F, et al. Handb Clin Neurol. 2017; 144: 63 -67.

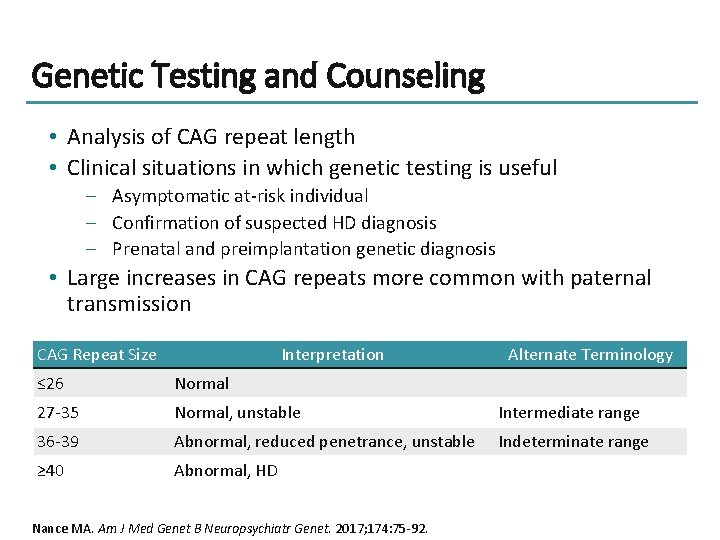
Genetic Testing and Counseling • Analysis of CAG repeat length • Clinical situations in which genetic testing is useful – Asymptomatic at-risk individual – Confirmation of suspected HD diagnosis – Prenatal and preimplantation genetic diagnosis • Large increases in CAG repeats more common with paternal transmission CAG Repeat Size Interpretation Alternate Terminology ≤ 26 Normal 27 -35 Normal, unstable Intermediate range 36 -39 Abnormal, reduced penetrance, unstable Indeterminate range ≥ 40 Abnormal, HD Nance MA. Am J Med Genet B Neuropsychiatr Genet. 2017; 174: 75 -92.

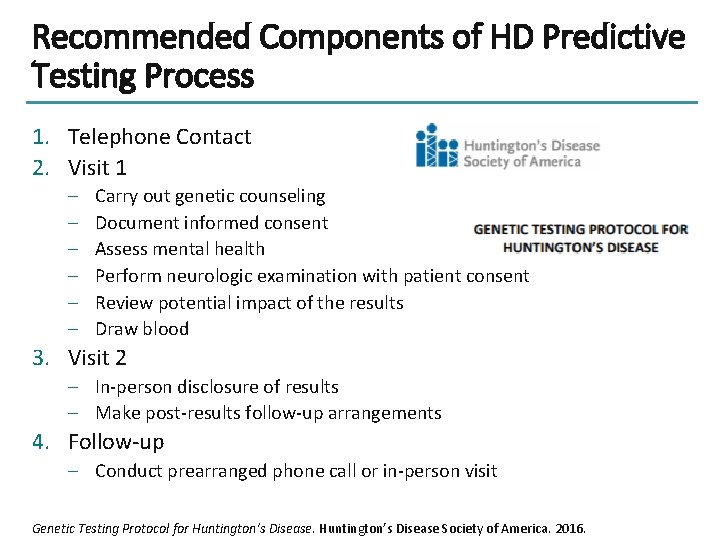
Recommended Components of HD Predictive Testing Process 1. Telephone Contact 2. Visit 1 – – – Carry out genetic counseling Document informed consent Assess mental health Perform neurologic examination with patient consent Review potential impact of the results Draw blood 3. Visit 2 – In-person disclosure of results – Make post-results follow-up arrangements 4. Follow-up – Conduct prearranged phone call or in-person visit Genetic Testing Protocol for Huntington’s Disease Society of America. 2016.

General Management Strategies • Identify most bothersome symptoms by interviewing patient and caregiver • Address each bothersome symptom with the most appropriate strategy – Neuro-driven therapy § Physical therapy § Occupational therapy § Speech therapy – Pharmacotherapy – Psychotherapy or counseling – Complementary medicine Nance M, et al. A Physician’s Guide to the Management of Huntington’s Disease. 2011.

Treating Psychiatric Symptoms: Nonpharmacologic Approaches Multidisciplinary Team Nonpharmacologic Approaches • Neuropsychiatrist • Psychologist • Social worker • Identify and alleviate potential external drivers of mood changes • Counseling • Mindfulness training or meditation Education • Patient • Family and caregivers • Community Nance M, et al. A Physician’s Guide to the Management of Huntington’s Disease. 2011.

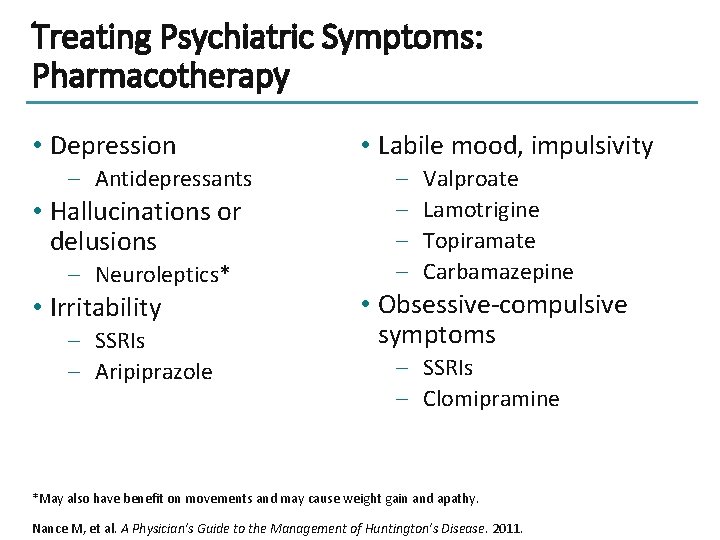
Treating Psychiatric Symptoms: Pharmacotherapy • Depression – Antidepressants • Hallucinations or delusions – Neuroleptics* • Irritability – SSRIs – Aripiprazole • Labile mood, impulsivity – – Valproate Lamotrigine Topiramate Carbamazepine • Obsessive-compulsive symptoms – SSRIs – Clomipramine *May also have benefit on movements and may cause weight gain and apathy. Nance M, et al. A Physician’s Guide to the Management of Huntington’s Disease. 2011.
![Treating Cognitive Symptoms Neural compensation seen in preclinical stagesa No accepted cognitive Treating Cognitive Symptoms • Neural compensation seen in preclinical stages[a] • No accepted cognitive](https://slidetodoc.com/presentation_image/0dc534486e4b8b9975facdbea2f0055e/image-15.jpg)
Treating Cognitive Symptoms • Neural compensation seen in preclinical stages[a] • No accepted cognitive battery[b] – Mo. CA may be useful • Break tasks into smaller steps or use checklist[c] • Ensure patient participates in activities and uses new information[c] a. Papoutsi M, et al. Mov Disord. 2014; 29: 673 -683; b. Paulsen JS. Curr Neurol Neurosci Rep. 2011; 11: 474 -483; c. Johnson AC, et al. Understanding Behavior in Huntington's Disease: A Guide for Professionals; 2014.

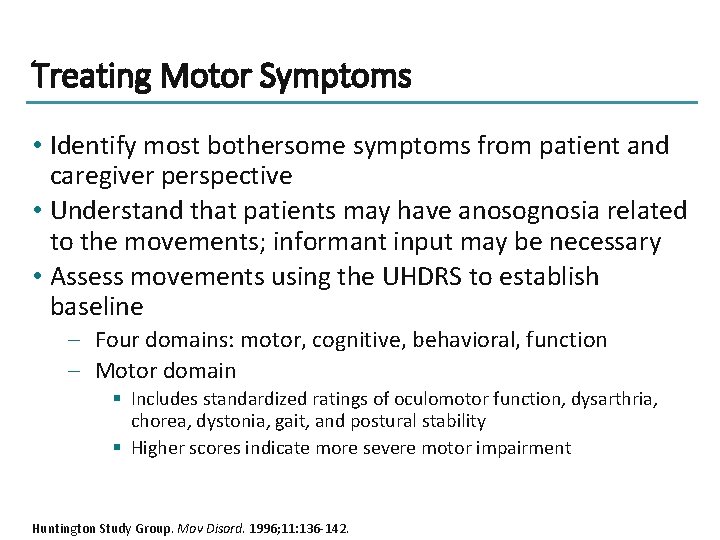
Treating Motor Symptoms • Identify most bothersome symptoms from patient and caregiver perspective • Understand that patients may have anosognosia related to the movements; informant input may be necessary • Assess movements using the UHDRS to establish baseline – Four domains: motor, cognitive, behavioral, function – Motor domain § Includes standardized ratings of oculomotor function, dysarthria, chorea, dystonia, gait, and postural stability § Higher scores indicate more severe motor impairment Huntington Study Group. Mov Disord. 1996; 11: 136 -142.
![Treating Chorea Treat chorea to Improve quality of lifea b Prevent Treating Chorea • Treat chorea to – Improve quality of life[a, b] – Prevent](https://slidetodoc.com/presentation_image/0dc534486e4b8b9975facdbea2f0055e/image-17.jpg)
Treating Chorea • Treat chorea to – Improve quality of life[a, b] – Prevent injuries[b] – Increase daily functioning at work and home[a, b] • Educate patients and caregivers – About purpose of treatment[b] – The impact of chorea on functioning[b]* § Tripping § Swallowing problems § Dropping things *Manifestations of chorea may include these and other indicators a. Jankovic J, et al. Mov Disord. 2014; 29: 1414 -1418; b. Coppen EM, et al. Drugs. 2017; 77: 29 -46.
![Pharmacologic Treatment of Chorea Associated With HD FDAapproved drugs Tetrabenazinea and deutetrabenazineb Pharmacologic Treatment of Chorea Associated With HD • FDA-approved drugs – Tetrabenazine[a] and deutetrabenazine[b]](https://slidetodoc.com/presentation_image/0dc534486e4b8b9975facdbea2f0055e/image-18.jpg)
Pharmacologic Treatment of Chorea Associated With HD • FDA-approved drugs – Tetrabenazine[a] and deutetrabenazine[b] § VMAT-2 inhibitors § Include boxed warning for increased risk of depression and suicidality • Off-label treatments – Neuroleptics[c] – Benzodiazepines[c] – Levitiracetam[d] a. Xenazine® PI 2008; b. Austedo® PI 2017; c. Coppen EM, et al. Drugs. 2017; 77: 29 -46; d. Zesiewicz TA, et al. Mov Disord. 2006; 21: 1998 -2001.
![Tetrabenazine and Deutetrabenazine Parameter Approval Pharmacokineticsa Tetrabenazine Deutetrabenazine 2008 2017 P Cmax ngm L Tetrabenazine and Deutetrabenazine Parameter Approval Pharmacokinetics[a] Tetrabenazine Deutetrabenazine 2008 2017 P Cmax, ng/m. L](https://slidetodoc.com/presentation_image/0dc534486e4b8b9975facdbea2f0055e/image-19.jpg)
Tetrabenazine and Deutetrabenazine Parameter Approval Pharmacokinetics[a] Tetrabenazine Deutetrabenazine 2008 2017 P Cmax, ng/m. L 65. 1 22. 5 Tmax, h 1. 0 2. 3 T 1/2, h 4. 5 9. 4 Dosing regimen 3 X daily 2 x daily Serious AEs* 5. 44 (0. 28, 104. 49) 1. 00 (0. 06, 16. 50) Depression* 11. 15 (0. 62, 200. 33) 0. 65 (0. 10, 4. 10) UHDRS chorea score -3. 5 (-5. 2, -1. 9) -2. 5 (-3. 7, -1. 3) UHDRS total motor score -3. 3 (-7. 0, 0. 3) -4. 0 (-6. 5, -1. 5) Pivotal study results[b] *Odds ratio vs placebo (95% confidence interval) in clinical trials. a. Stamler D, et al. Mov Disord. 2013; 28(Suppl 1): 765; b. Rodrigues FB, et al. Mov Disord. 2017; 4: 582 -585.

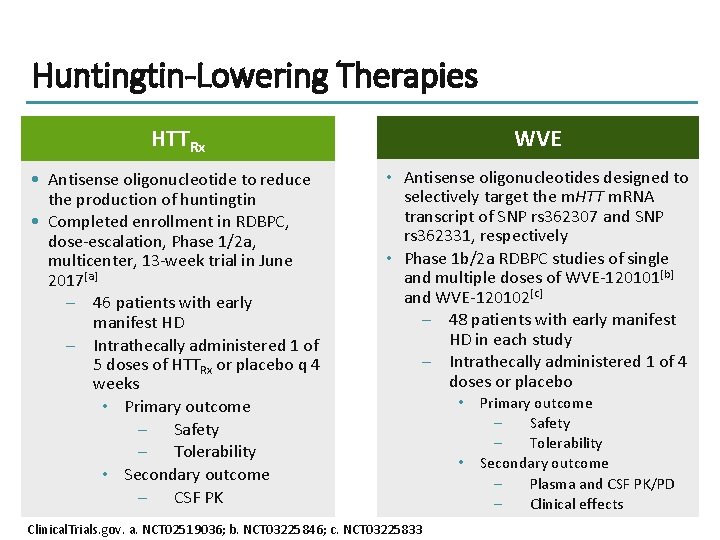
Huntingtin-Lowering Therapies HTTRx WVE • Antisense oligonucleotide to reduce the production of huntingtin • Completed enrollment in RDBPC, dose-escalation, Phase 1/2 a, multicenter, 13 -week trial in June 2017[a] – 46 patients with early manifest HD – Intrathecally administered 1 of 5 doses of HTTRx or placebo q 4 weeks • Primary outcome – Safety – Tolerability • Secondary outcome – CSF PK • Antisense oligonucleotides designed to selectively target the m. HTT m. RNA transcript of SNP rs 362307 and SNP rs 362331, respectively • Phase 1 b/2 a RDBPC studies of single and multiple doses of WVE-120101[b] and WVE-120102[c] – 48 patients with early manifest HD in each study – Intrathecally administered 1 of 4 doses or placebo Clinical. Trials. gov. a. NCT 02519036; b. NCT 03225846; c. NCT 03225833 • • Primary outcome – Safety – Tolerability Secondary outcome – Plasma and CSF PK/PD – Clinical effects

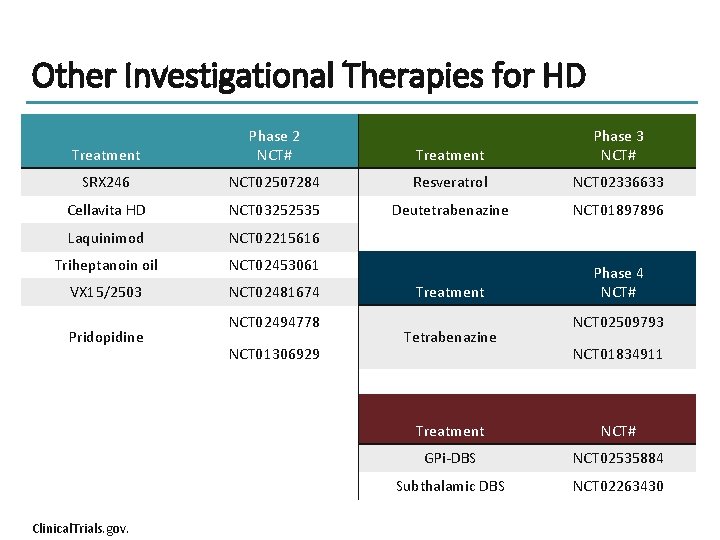
Other Investigational Therapies for HD Treatment Phase 2 NCT# Treatment Phase 3 NCT# SRX 246 NCT 02507284 Resveratrol NCT 02336633 Cellavita HD NCT 03252535 Deutetrabenazine NCT 01897896 Laquinimod NCT 02215616 Triheptanoin oil NCT 02453061 VX 15/2503 NCT 02481674 Treatment Phase 4 NCT# Pridopidine Clinical. Trials. gov. NCT 02494778 NCT 01306929 Tetrabenazine NCT 02509793 NCT 01834911 Treatment NCT# GPi-DBS NCT 02535884 Subthalamic DBS NCT 02263430

Clinical Trial Participation • Many new therapies are emerging and require participants for clinical trials – HDSA can assist in matching participants with appropriate trials through HDTrialfinder. org • Participation in a trial – Does not mean that patients will not receive excellent care – Required to ensure new therapies can move forward • Clinicians must address patient concerns and understand why they are not enrolling

Conclusion • HD is an autosomal dominant disease characterized by a CAG repeat – ≥ 40 repeats is positive for HD • Symptoms are motor (especially chorea), cognitive, and psychiatric/behavioral – Can include lack of executive functioning, labile mood, depression, impulsivity, and hallucinations • Management strategies for chorea include FDAapproved VMAT-2 inhibitors

Thank you for participating in this activity. To proceed to the online CME test, click on the Earn Credit link on this page.
 Is down syndrome autosomal or sexlinked
Is down syndrome autosomal or sexlinked Communicable disease and non communicable disease
Communicable disease and non communicable disease Samuel huntington
Samuel huntington Aaliyah huntington
Aaliyah huntington Huntington postulates
Huntington postulates Athletic clearance.com login
Athletic clearance.com login Samuel huntington biographie
Samuel huntington biographie Acondrolasia
Acondrolasia George huntington
George huntington Clash of civilizations huntington
Clash of civilizations huntington Doença de huntington
Doença de huntington Modafinil price costco
Modafinil price costco Clash of civilizations huntington
Clash of civilizations huntington Huntington's postulates
Huntington's postulates Pma psikiyatri
Pma psikiyatri Postulados de huntington
Postulados de huntington Desktop activity moderator driver
Desktop activity moderator driver Liking scale 1-5
Liking scale 1-5 Moderator examples
Moderator examples Constant moderator
Constant moderator Moderator speaker
Moderator speaker Mediator versus moderator
Mediator versus moderator Cara moderator
Cara moderator Yang hu lancaster
Yang hu lancaster Interfering variable
Interfering variable