HPTN 094 INTEGRA LA County Site HPTN 094











- Slides: 16

HPTN 094: INTEGRA LA County Site

HPTN 094 Overview Title: A Vanguard Study of Health Service Delivery in a Mobile Health Delivery Unit to Link Persons who Inject Drugs to Integrated Care and Prevention for Addiction, HIV, HCV and Primary Care Design: Two-arm, individually randomized, controlled, open label study Study duration: Approximately 3. 5 years total Individual participants in study for approximately 52 weeks Sites: Los Angeles, Houston, New York, Philadelphia, and Washington, D. C.

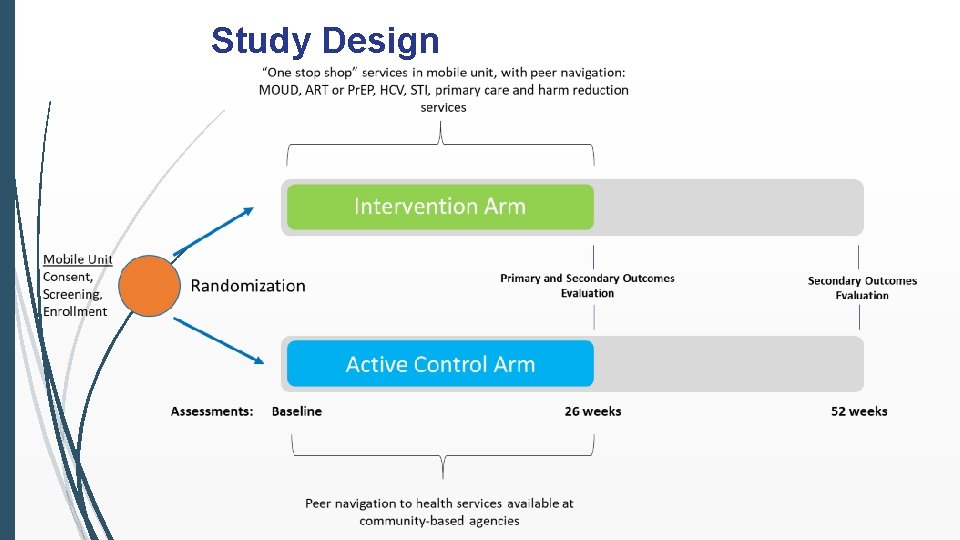
Study Design



Participants 860 total participants (~170 per site) allotted 1: 1 to intervention and active control arms Targets: 25% women 25% participants under 30 years of age 460 HIV-positive and 400 HIVnegative participants

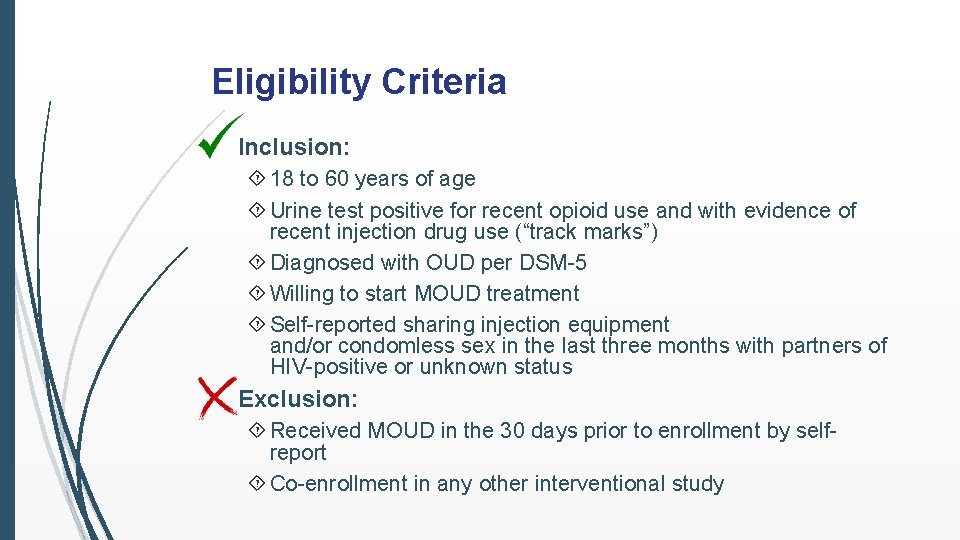
Eligibility Criteria Inclusion: 18 to 60 years of age Urine test positive for recent opioid use and with evidence of recent injection drug use (“track marks”) Diagnosed with OUD per DSM-5 Willing to start MOUD treatment Self-reported sharing injection equipment and/or condomless sex in the last three months with partners of HIV-positive or unknown status Exclusion: Received MOUD in the 30 days prior to enrollment by selfreport Co-enrollment in any other interventional study

Study Visits Screening and Enrollment Visits: All potential study participants will provide biological samples and selfreported data via interview in the mobile unit Samples will be tested for HIV, HAV, HBV, HCV, and STIs 26 and 52 week visits: All participants (both arms) will have study visits at 26 and 52 weeks for evaluation of study outcomes

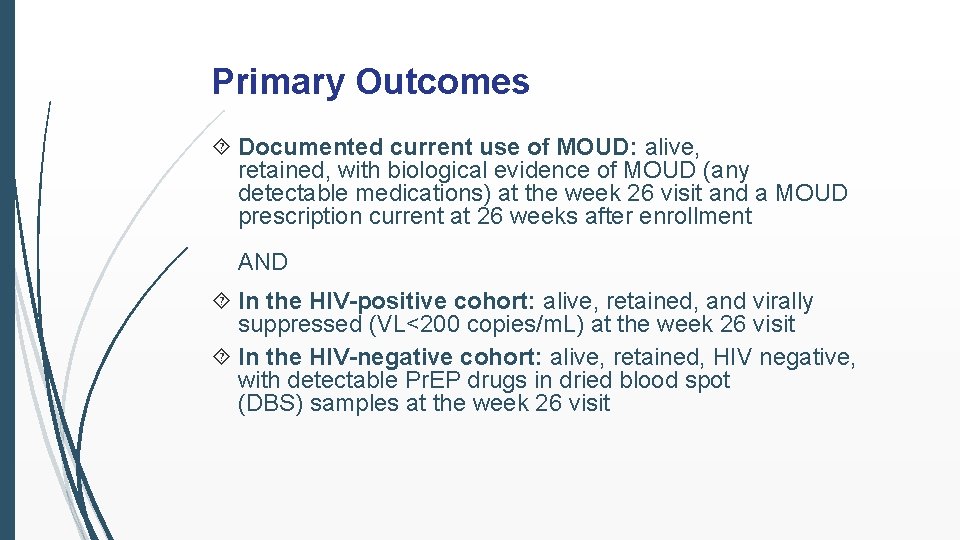
Primary Outcomes Documented current use of MOUD: alive, retained, with biological evidence of MOUD (any detectable medications) at the week 26 visit and a MOUD prescription current at 26 weeks after enrollment AND In the HIV-positive cohort: alive, retained, and virally suppressed (VL<200 copies/m. L) at the week 26 visit In the HIV-negative cohort: alive, retained, HIV negative, with detectable Pr. EP drugs in dried blood spot (DBS) samples at the week 26 visit

Implementation

Timeline Pre-Implementation (Fall 2020 -Spring 2021) Post-Implementation (Fall 2024 -? ) Implementation (Spring 2021 -Fall 2024) Staggered implementation at different locations around LA County

Determining Locations Available maps: LAC HIV Surveillance Report California Opioid Dashboard overdose death counts/rates Homeless counts and 311 calls for encampments “Drive by” assessments to determine population and logistical spaces to set up shop Engagement with community service providers


Stakeholder Engagement LAC Public Health Agencies Community Medical Providers Political Leaders HPTN 094 Community Advisory Board Housing Providers Substance Use Providers

Next Steps Continue to engage with community partners Exchange information and expertise Build referral partnerships Set up 1 on 1 calls Continue location assessments Implement HPTN 094!

Questions? Contact the Los Angeles HPTN 094 study team at Hptn 094@mednet. ucla. edu
 0 94
0 94 Hptn 082
Hptn 082 Integra dundović servis
Integra dundović servis Re integra reglen
Re integra reglen Aftalers ugyldighed
Aftalers ugyldighed Modelo 123 dividendos exentos
Modelo 123 dividendos exentos Como se integra el sistema nacional anticorrupcion
Como se integra el sistema nacional anticorrupcion Iea integra 2020
Iea integra 2020 Plataforma escuela industrial 20 de julio
Plataforma escuela industrial 20 de julio Integra o sistema financeiro nacional
Integra o sistema financeiro nacional La federación se integra por entidades mediante
La federación se integra por entidades mediante Re integra reglen
Re integra reglen Hot site cold site warm site disaster recovery
Hot site cold site warm site disaster recovery Phenylalanine
Phenylalanine What is site inventory
What is site inventory Untangle vpn setup
Untangle vpn setup Howard county young authors contest
Howard county young authors contest