Homework Essay Exam HW 10 Chap 16 Conceptual







- Slides: 33

Homework, Essay, Exam HW 10: Chap 16: - Conceptual # 7, 10 Problem # 1 Due Nov 29 th Essay outlines returned Monday. Essay due Dec 8 th Hour Exam 3: Wednesday, November 29 th • In-class, Quantum Physics and Nuclear Physics • Twenty multiple-choice questions • Will cover: Chapters 13, 14, 15 and 16 Lecture material • You should bring – 1 page notes, written single sided – #2 Pencil and a Calculator – Review Monday November 27 th – Review test will be available online on Monday Phy 107 Fall 2006 1

From the Last Time • Radioactive decay: alpha, beta, gamma • Radioactive half-life • Decay types understood in terms of number neutrons, protons and size of the nucleus. • Beta decays due to the weak force Today: Fission and Fusion Phy 107 Fall 2006 2

Other carbon decays • Lightest isotopes of carbon are observed to emit a particle like an electron, but has a positive charge! • This is the antiparticle of the electron. • Called the positron. Phy 107 Fall 2006 3

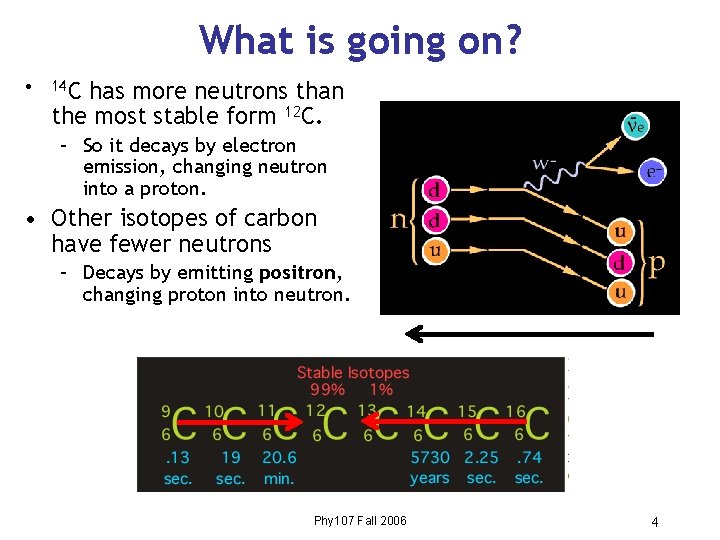
What is going on? • 14 C has more neutrons than the most stable form 12 C. – So it decays by electron emission, changing neutron into a proton. • Other isotopes of carbon have fewer neutrons – Decays by emitting positron, changing proton into neutron. Phy 107 Fall 2006 4

Gamma decay • So far – Alpha decay: alpha particle emitted from nucleus – Beta decay: electron or positron emitted • Both can leave the nucleus in excited state – Just like a hydrogen atom can be in an excited state – Hydrogen emits photon as it drops to lower state. Nucleus also emits photon as it drops to ground state This is gamma radiation But energies much larger, so extremely high energy photons. Phy 107 Fall 2006 5

Turning lead into gold Radioactive decay changes one element into another by changing the number of protons in a nucleus. This can also be done artificially by neutron bombardment. • The transmutation of platinum into gold accomplished by a sequence of two nuclear reactions • first: 198 Pt + neutron --> 199 Pt • second: 199 Pt --> 199 Au + subatomic particle Phy 107 Fall 2006 6

Radioactive decay summary • Alpha decay – Nucleus emits alpha particle (2 neutrons + 2 protons) – Happens with heavy nuclei only – Caused by Coulomb repulsion • Beta decay – Nucleus emits electron (beta-) or positron (beta+) – Internally, neutron changes to proton (beta-), or proton changes to neutron (beta+) – Caused by weak force • Gamma decay – Nucleus starts in internal excited state – Emits photon and drops to lower energy state Phy 107 Fall 2006 7

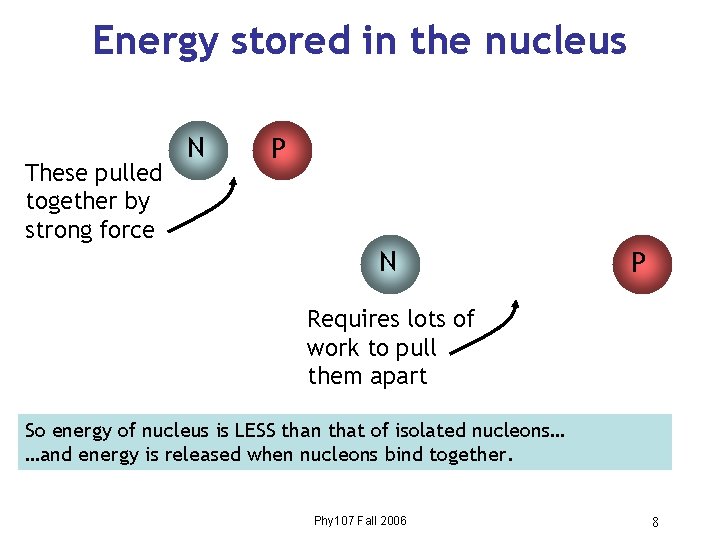
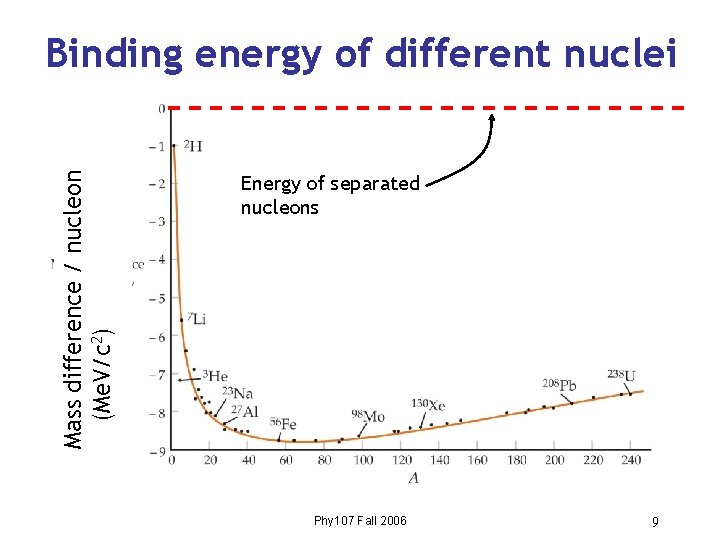
Energy stored in the nucleus These pulled together by strong force N P Requires lots of work to pull them apart So energy of nucleus is LESS than that of isolated nucleons… …and energy is released when nucleons bind together. Phy 107 Fall 2006 8

Mass difference / nucleon (Me. V/c 2) Binding energy of different nuclei Energy of separated nucleons Phy 107 Fall 2006 9

Energy Production How can we release this energy? Phy 107 Fall 2006 10

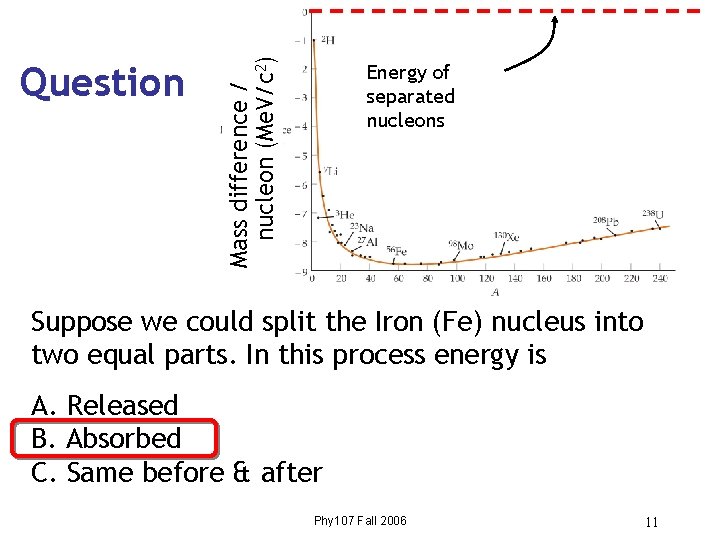
Mass difference / nucleon (Me. V/c 2) Question Energy of separated nucleons Suppose we could split the Iron (Fe) nucleus into two equal parts. In this process energy is A. Released B. Absorbed C. Same before & after Phy 107 Fall 2006 11

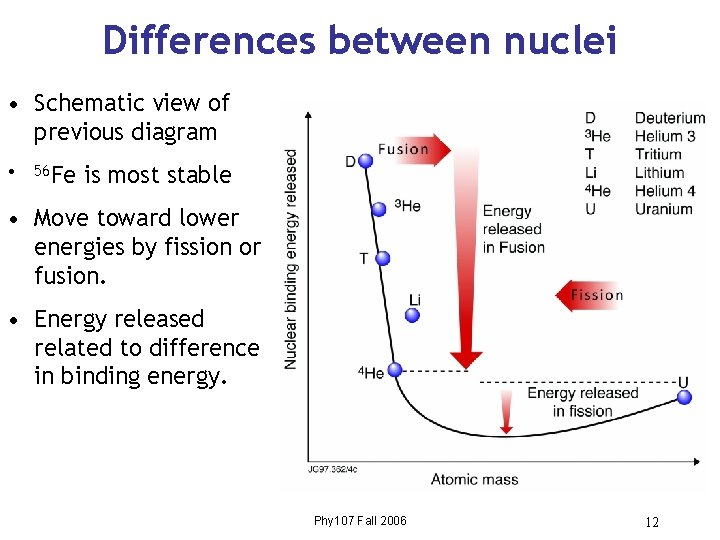
Differences between nuclei • Schematic view of previous diagram • 56 Fe is most stable • Move toward lower energies by fission or fusion. • Energy released related to difference in binding energy. Phy 107 Fall 2006 12

Nuclear fission • A heavy nucleus is split apart into two smaller ones. • Energy is released because the lighter nuclei are more tightly bound, less mass • E=mc 2, energy is released Phy 107 Fall 2006 13

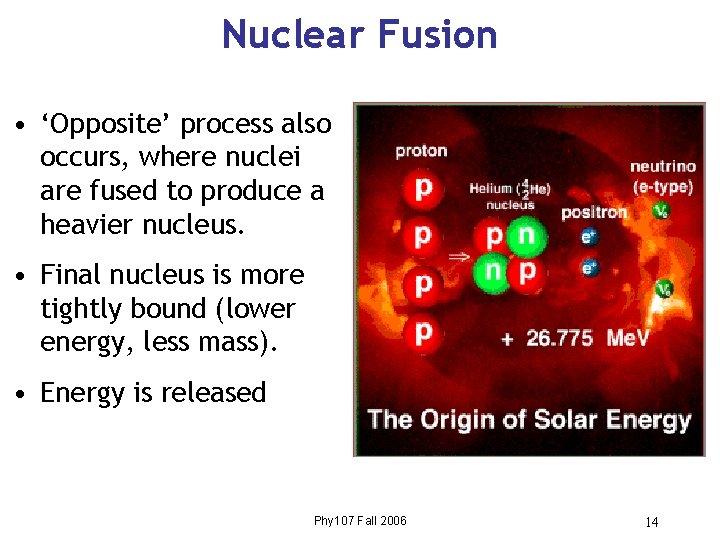
Nuclear Fusion • ‘Opposite’ process also occurs, where nuclei are fused to produce a heavier nucleus. • Final nucleus is more tightly bound (lower energy, less mass). • Energy is released Phy 107 Fall 2006 14

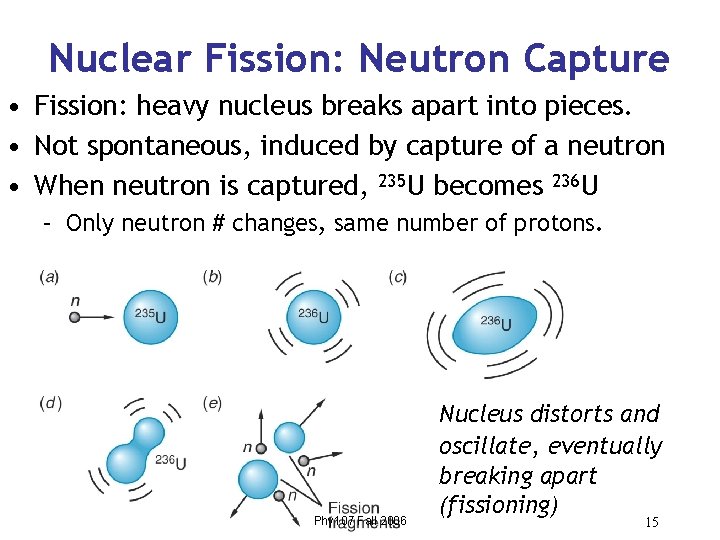
Nuclear Fission: Neutron Capture • Fission: heavy nucleus breaks apart into pieces. • Not spontaneous, induced by capture of a neutron • When neutron is captured, 235 U becomes 236 U – Only neutron # changes, same number of protons. Phy 107 Fall 2006 Nucleus distorts and oscillate, eventually breaking apart (fissioning) 15

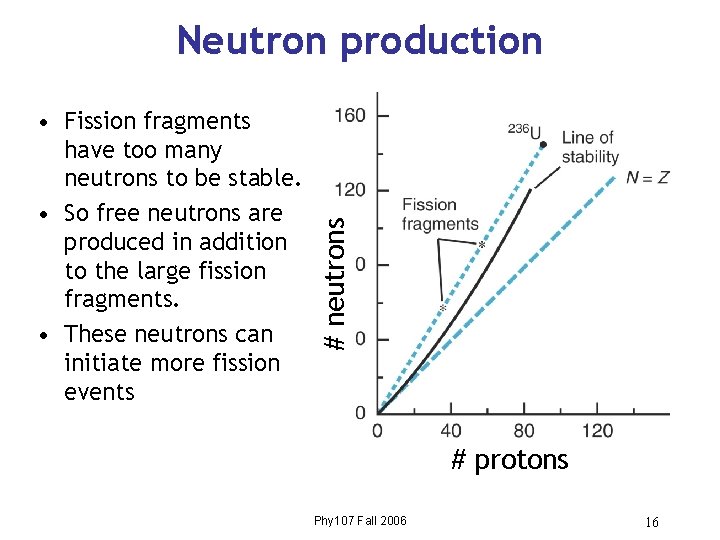
• Fission fragments have too many neutrons to be stable. • So free neutrons are produced in addition to the large fission fragments. • These neutrons can initiate more fission events # neutrons Neutron production # protons Phy 107 Fall 2006 16

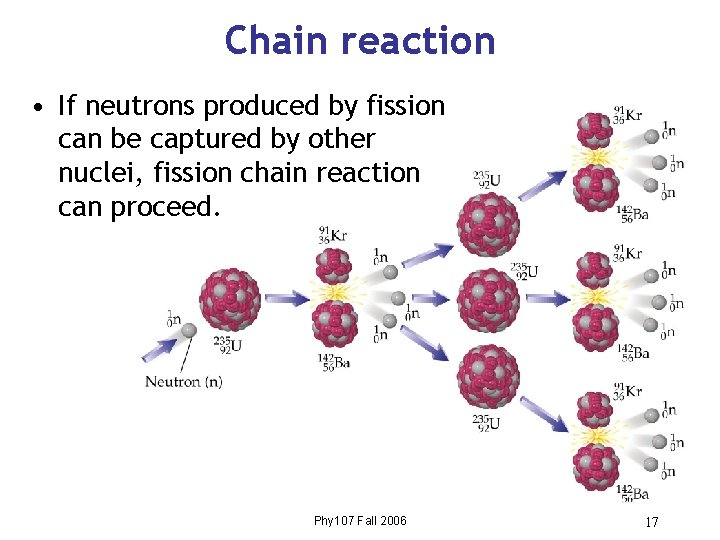
Chain reaction • If neutrons produced by fission can be captured by other nuclei, fission chain reaction can proceed. Phy 107 Fall 2006 17

Neutrons • Neutrons may be captured by nuclei that do not undergo fission – Most commonly, neutrons are captured by 238 U – The possibility of neutron capture by 238 U is lower for slow neutrons. • The moderator helps minimize the capture of neutrons by 238 U by slowing them down, making more available to initiate fission in 235 U. Phy 107 Fall 2006 18

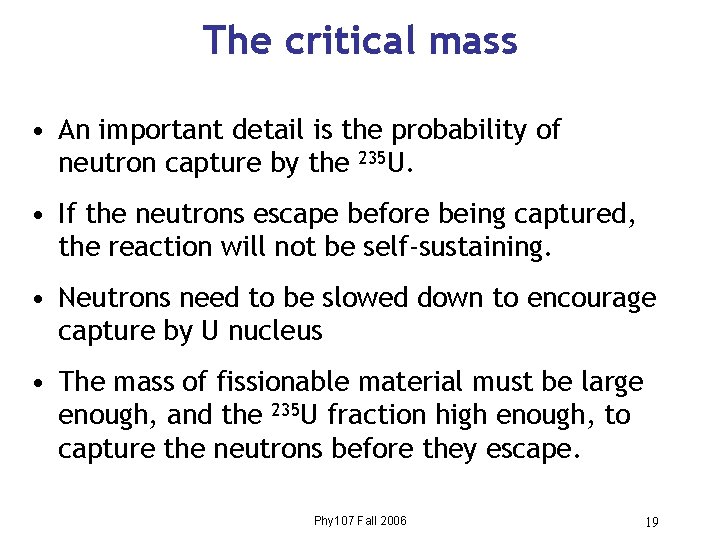
The critical mass • An important detail is the probability of neutron capture by the 235 U. • If the neutrons escape before being captured, the reaction will not be self-sustaining. • Neutrons need to be slowed down to encourage capture by U nucleus • The mass of fissionable material must be large enough, and the 235 U fraction high enough, to capture the neutrons before they escape. Phy 107 Fall 2006 19

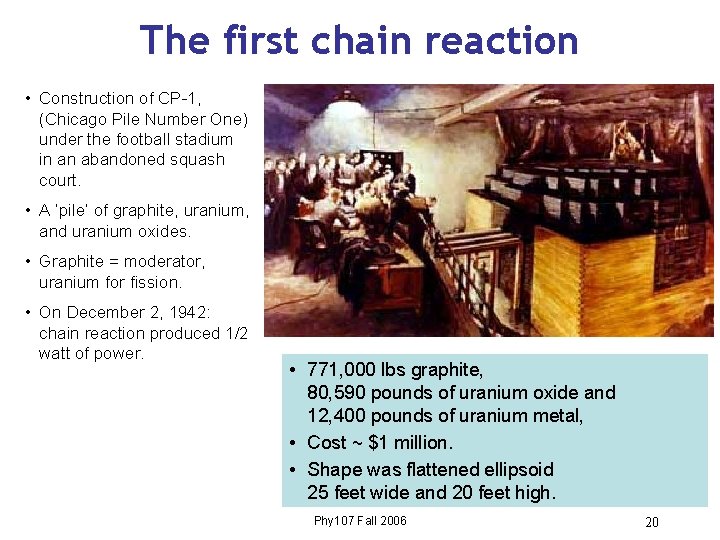
The first chain reaction • Construction of CP-1, (Chicago Pile Number One) under the football stadium in an abandoned squash court. • A ‘pile’ of graphite, uranium, and uranium oxides. • Graphite = moderator, uranium for fission. • On December 2, 1942: chain reaction produced 1/2 watt of power. • 771, 000 lbs graphite, 80, 590 pounds of uranium oxide and 12, 400 pounds of uranium metal, • Cost ~ $1 million. • Shape was flattened ellipsoid 25 feet wide and 20 feet high. Phy 107 Fall 2006 20

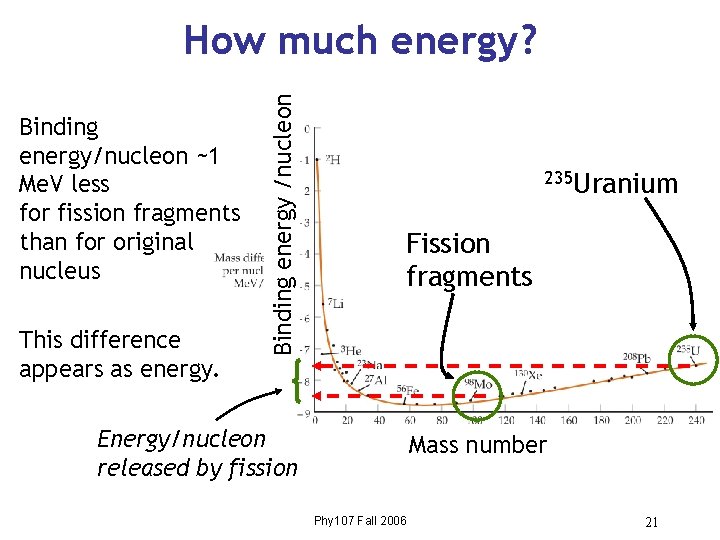
Binding energy/nucleon ~1 Me. V less for fission fragments than for original nucleus This difference appears as energy. Binding energy /nucleon How much energy? 235 Uranium Fission fragments Energy/nucleon released by fission Mass number Phy 107 Fall 2006 21

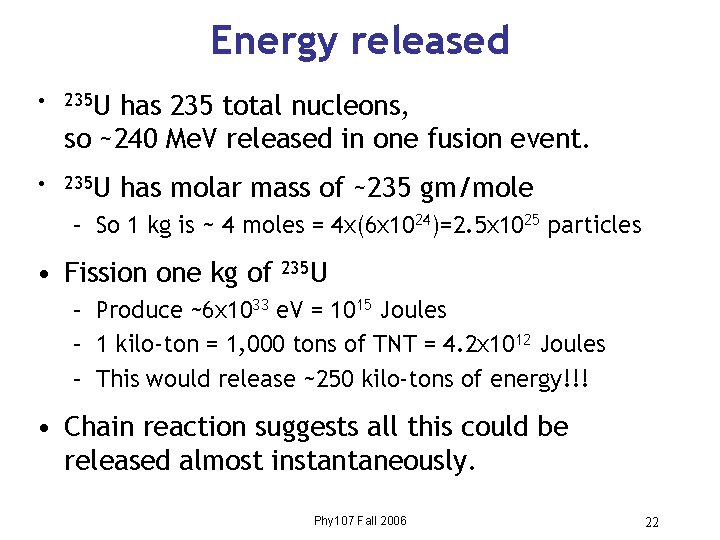
Energy released • 235 U has 235 total nucleons, so ~240 Me. V released in one fusion event. • 235 U has molar mass of ~235 gm/mole – So 1 kg is ~ 4 moles = 4 x(6 x 1024)=2. 5 x 1025 particles • Fission one kg of 235 U – Produce ~6 x 1033 e. V = 1015 Joules – 1 kilo-ton = 1, 000 tons of TNT = 4. 2 x 1012 Joules – This would release ~250 kilo-tons of energy!!! • Chain reaction suggests all this could be released almost instantaneously. Phy 107 Fall 2006 22

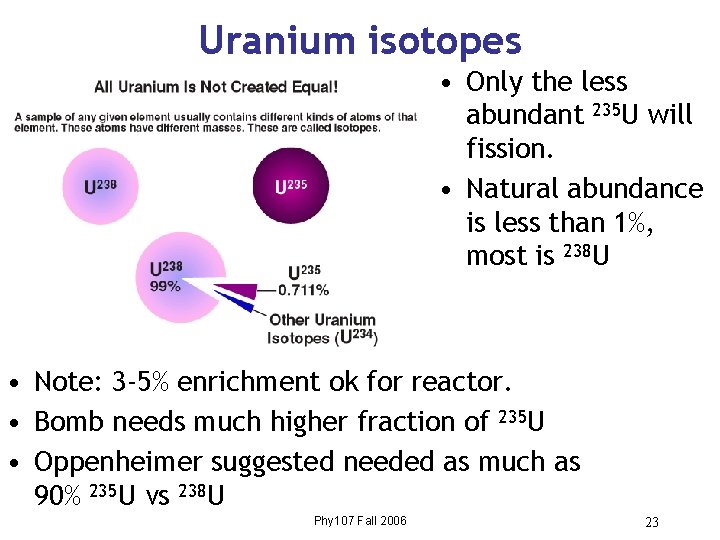
Uranium isotopes • Only the less abundant 235 U will fission. • Natural abundance is less than 1%, most is 238 U • Note: 3 -5% enrichment ok for reactor. • Bomb needs much higher fraction of 235 U • Oppenheimer suggested needed as much as 90% 235 U vs 238 U Phy 107 Fall 2006 23

Where does uranium come from? • Uranium is abundant, but in low concentration • E. g. uranium is mixed with granite, covering 60% of the Earth’s crust. • But only four parts of uranium per million parts of granite. Phy 107 Fall 2006 24

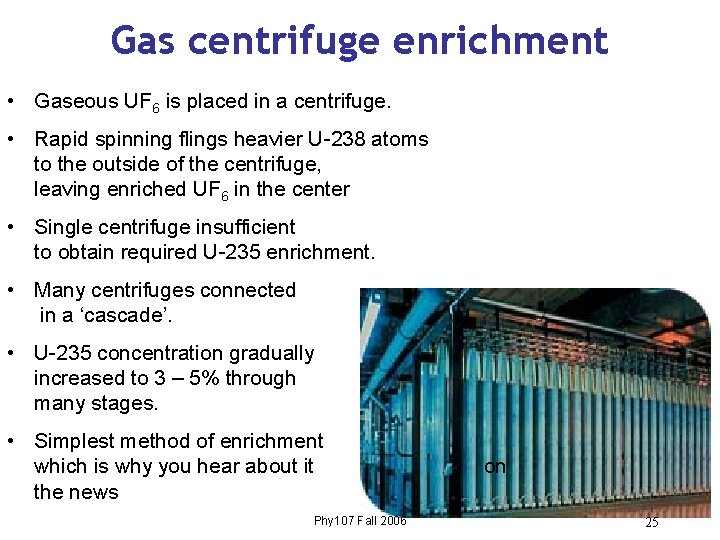
Gas centrifuge enrichment • Gaseous UF 6 is placed in a centrifuge. • Rapid spinning flings heavier U-238 atoms to the outside of the centrifuge, leaving enriched UF 6 in the center • Single centrifuge insufficient to obtain required U-235 enrichment. • Many centrifuges connected in a ‘cascade’. • U-235 concentration gradually increased to 3 – 5% through many stages. • Simplest method of enrichment which is why you hear about it the news Phy 107 Fall 2006 on 25

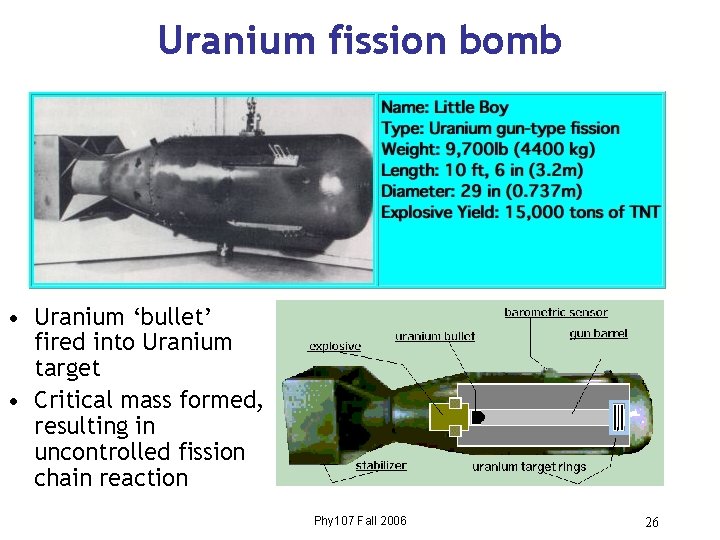
Uranium fission bomb • Uranium ‘bullet’ fired into Uranium target • Critical mass formed, resulting in uncontrolled fission chain reaction Phy 107 Fall 2006 26

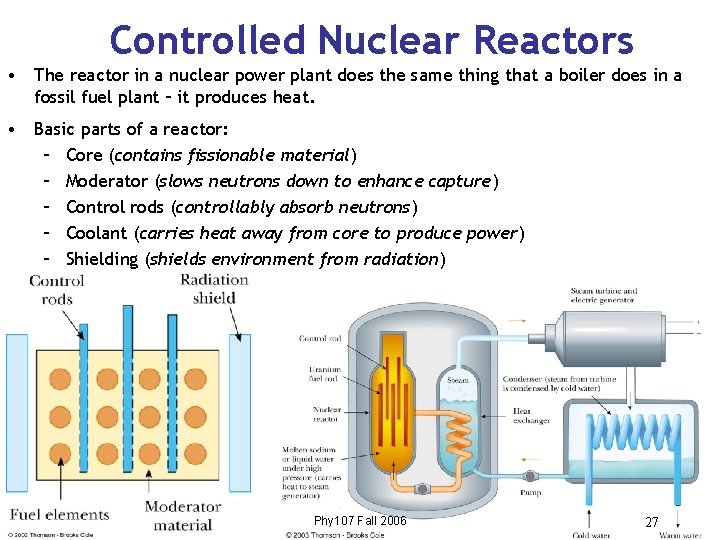
Controlled Nuclear Reactors • The reactor in a nuclear power plant does the same thing that a boiler does in a fossil fuel plant – it produces heat. • Basic parts of a reactor: – Core (contains fissionable material) – Moderator (slows neutrons down to enhance capture) – Control rods (controllably absorb neutrons) – Coolant (carries heat away from core to produce power) – Shielding (shields environment from radiation) Phy 107 Fall 2006 27

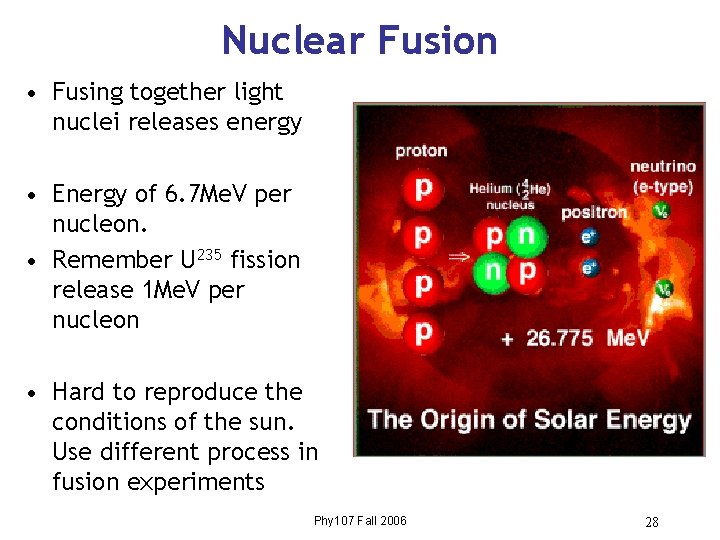
Nuclear Fusion • Fusing together light nuclei releases energy • Energy of 6. 7 Me. V per nucleon. • Remember U 235 fission release 1 Me. V per nucleon • Hard to reproduce the conditions of the sun. Use different process in fusion experiments Phy 107 Fall 2006 28

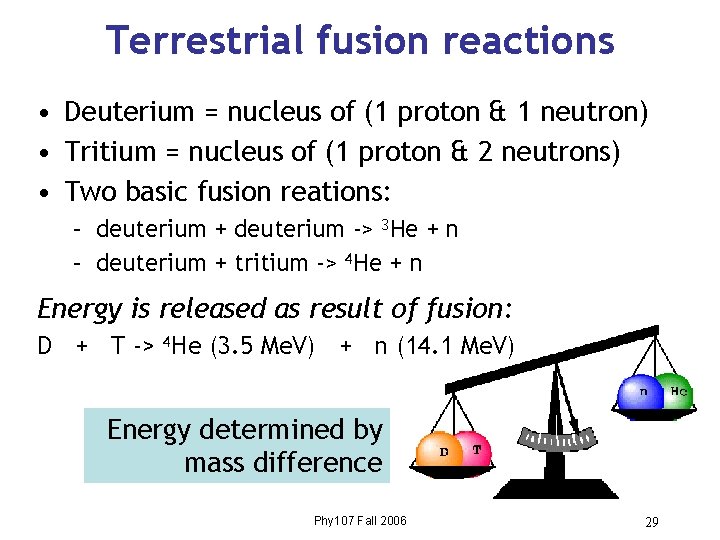
Terrestrial fusion reactions • Deuterium = nucleus of (1 proton & 1 neutron) • Tritium = nucleus of (1 proton & 2 neutrons) • Two basic fusion reations: – deuterium + deuterium -> 3 He + n – deuterium + tritium -> 4 He + n Energy is released as result of fusion: D + T -> 4 He (3. 5 Me. V) + n (14. 1 Me. V) Energy determined by mass difference Phy 107 Fall 2006 29

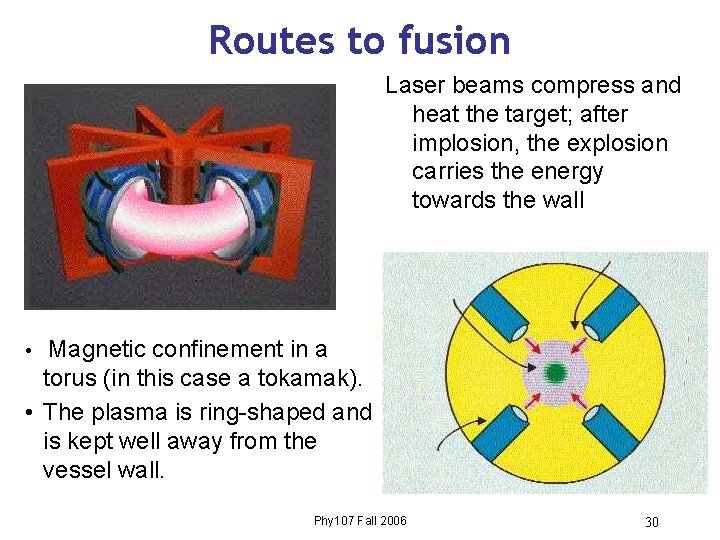
Routes to fusion Laser beams compress and heat the target; after implosion, the explosion carries the energy towards the wall Magnetic confinement in a torus (in this case a tokamak). • The plasma is ring-shaped and is kept well away from the vessel wall. • Phy 107 Fall 2006 30

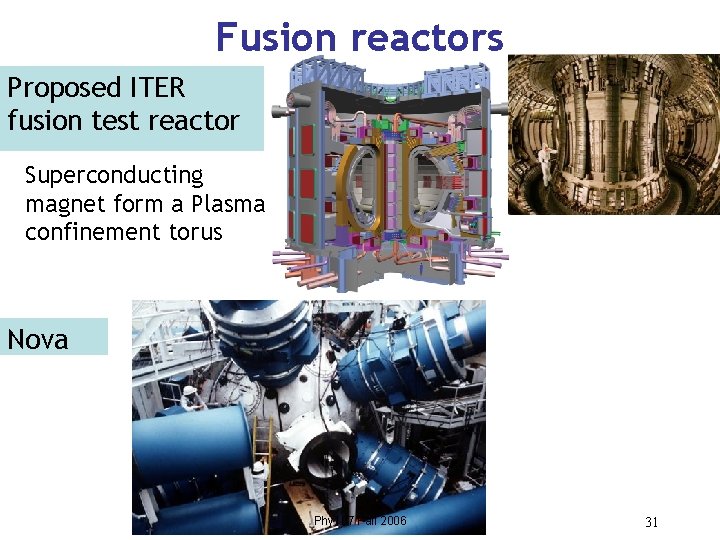
Fusion reactors Proposed ITER fusion test reactor Superconducting magnet form a Plasma confinement torus Nova Phy 107 Fall 2006 31

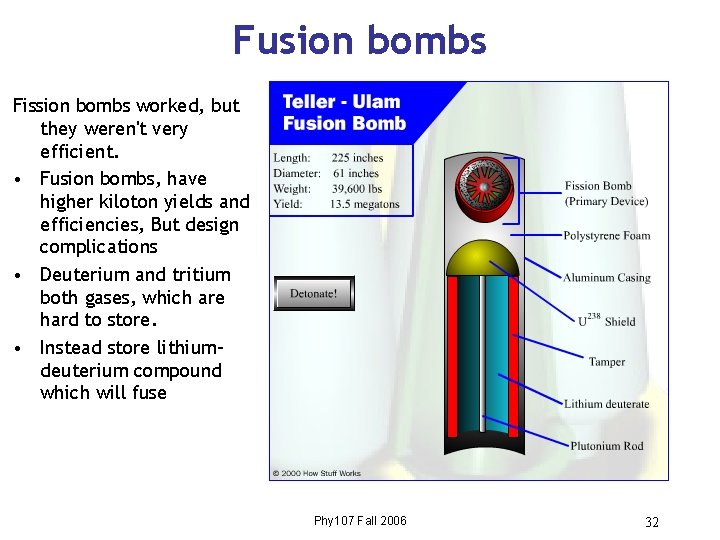
Fusion bombs Fission bombs worked, but they weren't very efficient. • Fusion bombs, have higher kiloton yields and efficiencies, But design complications • Deuterium and tritium both gases, which are hard to store. • Instead store lithiumdeuterium compound which will fuse Phy 107 Fall 2006 32

Fission and Fusion • Fission: – – Heavy nucleus is broken apart Total mass of pieces less than original nucleus Missing mass appears as energy E=mc 2 Radioactive decay products left over • Fusion – Light nuclei are fused together into heavier nuclei – Total mass of original nuclei greater than resulting nucleus – Missing mass appears as energy. Phy 107 Fall 2006 33
 Chap chap slide
Chap chap slide Homework oh homework i hate you you stink
Homework oh homework i hate you you stink Homework oh homework i hate you you stink
Homework oh homework i hate you you stink Parts of a poem
Parts of a poem Oh homework oh homework poem
Oh homework oh homework poem Alitteration definition
Alitteration definition Consonance
Consonance 1 of 1 clothing meaning
1 of 1 clothing meaning Passion chap 6
Passion chap 6 Bank run chap 11
Bank run chap 11 Durbin chap
Durbin chap I look like jeera
I look like jeera Kstn chap 18
Kstn chap 18 Close family - chapter 3
Close family - chapter 3 The origin of species chapter 1
The origin of species chapter 1 Satisfying needs 7
Satisfying needs 7 The origin of species chap 22
The origin of species chap 22 Find the passage
Find the passage Matthew 5
Matthew 5 Kinds in development chap 1
Kinds in development chap 1 Drivers of competitive behavior
Drivers of competitive behavior System engineer chap 1
System engineer chap 1 Chap tree
Chap tree Kstn chap 7
Kstn chap 7 I was in that state when a chap easily turns nasty analysis
I was in that state when a chap easily turns nasty analysis The origin of species chapter 24
The origin of species chapter 24 Passion chap 9
Passion chap 9 Hình cắt kết hợp
Hình cắt kết hợp In the summer chap 22
In the summer chap 22 Selection project 2
Selection project 2 Origin of species - chapter 18
Origin of species - chapter 18 Friendly relationship chapter 12
Friendly relationship chapter 12 Fitness - chapter 1
Fitness - chapter 1 Chap tree
Chap tree