High Throughput Donor Plasma NAT Screening Assay Applied








- Slides: 17

High Throughput Donor Plasma NAT Screening Assay Applied to Acute HIV Detection in a Public Health Setting December 5, 2007 SLIDE Josh Goldsmith, Ph. D. National Genetics Institute, a Lab. Corp Company

Presentation Outline § Current challenges with acute HIV screening § NGI PCR and pooling methods § A prospective clinical study to validate the method for plasma donor screening § Acute HIV demonstration project with SFDPH 2

Challenges in the Implementation of PCRBased Acute HIV Screening Programs § § Availability of validated methods Availability of “off-the-shelf” methods Availability of cost-effective methods Availability of scalable methods where broadbased screening programs are envisioned 3

National Genetics Institute (NGI) Profile § A provider of automated, sensitive PCR methods for infectious disease testing § Offers assays for HIV, HAV, HBV, HCV, and other infectious agents § Screens the majority of US supply of source plasma for infectious agents (~8 M donations/year) with pools of 512 samples § A licensed clinical laboratory and approved by FDA CBER to perform pooled nucleic acid testing for donor plasma screening § A subsidiary of Lab. Corp with access to blood draw centers and courier networks throughout the US 4

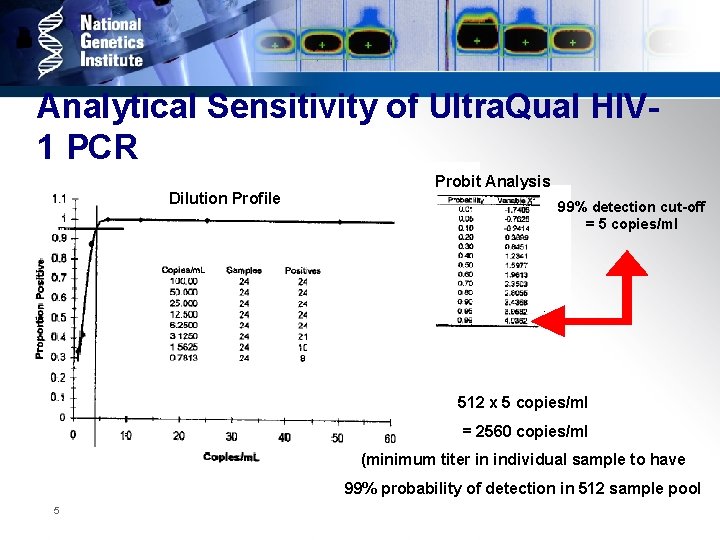
Analytical Sensitivity of Ultra. Qual HIV 1 PCR Dilution Profile Probit Analysis 99% detection cut-off = 5 copies/ml 512 x 5 copies/ml = 2560 copies/ml (minimum titer in individual sample to have 99% probability of detection in 512 sample pool 5

NGI Pooling and Pool Resolution Process § Launch Executable File 6

NGI Prospective Clinical Study Objectives § To demonstrate HIV-1 PCR testing of pooled plasma samples can accurately detect HIV-1 infection § To validate plasma pooling and pool resolution process for pools ≤ 512 individual samples § To determine the clinical sensitivity and specificity of the pooling and pool resolution process 7

NGI Prospective Clinical Study Design § 342, 729 plasma donations collected from ~48, 000 donors donating at 33 donor centers over a 3. 5 months § Informed consent obtained for HIV Ab and RT-PCR testing § Individual plasma samples tested for HIV Ab (Bio-Rad) and HIV-1 p 24 antigen (Coulter) and HIV RNA (NGI) 8

NGI Prospective Clinical Study Pool Numbers and Sizes 9

NGI Prospective Clinical Study Results 10

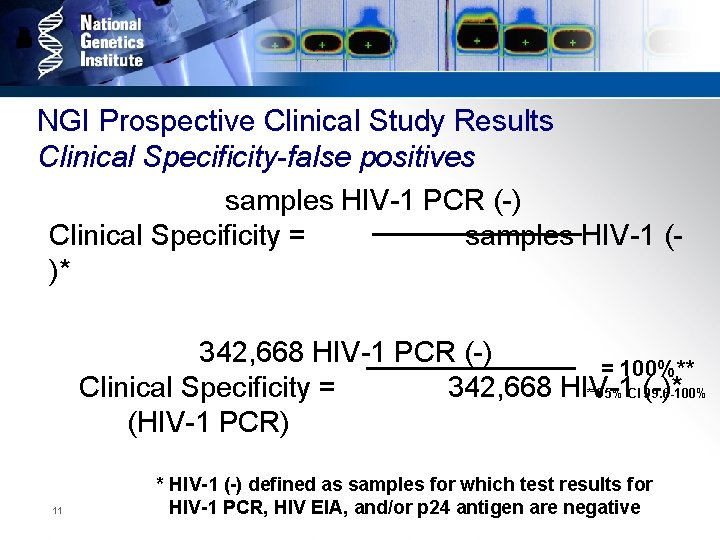
NGI Prospective Clinical Study Results Clinical Specificity-false positives samples HIV-1 PCR (-) Clinical Specificity = samples HIV-1 ()* 342, 668 HIV-1 PCR (-) = 100%** Clinical Specificity = 342, 668 HIV-1 **95% CI (-)* 99. 6 -100% (HIV-1 PCR) 11 * HIV-1 (-) defined as samples for which test results for HIV-1 PCR, HIV EIA, and/or p 24 antigen are negative

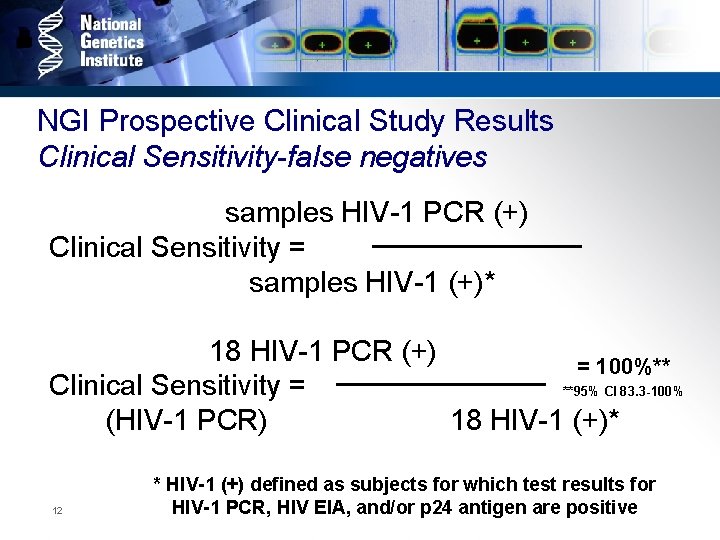
NGI Prospective Clinical Study Results Clinical Sensitivity-false negatives samples HIV-1 PCR (+) Clinical Sensitivity = samples HIV-1 (+)* 18 HIV-1 PCR (+) = 100%** Clinical Sensitivity = **95% CI 83. 3 -100% (HIV-1 PCR) 18 HIV-1 (+)* 12 * HIV-1 (+) defined as subjects for which test results for HIV-1 PCR, HIV EIA, and/or p 24 antigen are positive

NGI Prospective Clinical Study Conclusions § HIV-1 PCR testing of pooled plasma samples can accurately detect HIV-1 infection § Plasma pooling and pool resolution process for pools ≤ 512 individual samples is validated § Clinical sensitivity and specificity of the pooling and pool resolution process is high NGI received FDA biologics license from FDA (CBER) for screening of donated plasma based on the study results 13

San Francisco Department of Public Health (SFDPH) Demonstration Project - Study Overview 14 § Patient samples were collected from the SFDPH city clinics and community based services over a 5 -month period § Samples were screened by SFDPH for HIV antibodies with standard EIA (Vironostika) or rapid HIV antibody tests (Orasure Technologies) § HIV Ab (-) samples were picked up by Lab. Corp couriers and transported to NGI for testing § NAT positive samples were identified in pools of 64 samples and confirmed by individual NAT § All patients identified as positive were counseled and referred for HIV care and follow-up by SFDPH.

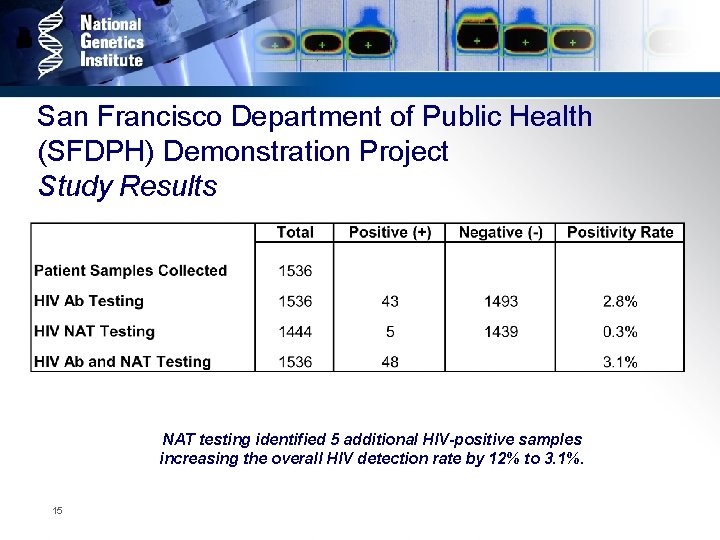
San Francisco Department of Public Health (SFDPH) Demonstration Project Study Results NAT testing identified 5 additional HIV-positive samples increasing the overall HIV detection rate by 12% to 3. 1%. 15

Conclusions § A pooled specimen HIV PCR assay has been validated for large-scale plasma donor screening involving pools of up to 512 samples § The method was applied to the detection of acute HIV in a public health setting with SFDPH § As this method is routinely used for screening millions of blood plasma donations per year, opportunities exist to implement broad-based acute HIV screening programs in a highly cost-effective fashion 16

Acknowledgements San Francisco Department of Public Health Jeff Klausner MD, MPH Nicola Zetola, Ph. D. Katherine Ahrens, MPH National Genetics Institute/Lab. Corp Mark Richardson, CLSp. MB Richard Smith, Ph. D. Hawazin Faruki, Ph. D. 17