High Intensity Focused Ultrasound HIFU for Liver Tumour







- Slides: 18

High Intensity Focused Ultrasound (HIFU) for Liver Tumour Dr Dai Wing Chiu Queen Mary Hospital

Background n Treatment options for hepatocellular carcinoma q q Resection Liver transplantation Local ablative therapy Transarterial chemoembolization (TACE)

Local Ablation Therapy n The first description of percutaneous ethanol injection (PEI) Liveraghi T et al. Radiology 1986 n Thermal techniques were first performed in the liver usingle bare tip neodymium yttrium aluminium garnet (Nd. YAG) laser fibres Steger A et al. BMJ 1989 n Five thermal techniques q q q Radiofrequency ablation Laser Microwave Cryotherapy High intensity focused ultrasound (HIFU)

High Intensity Focused Ultrasound n The first investigation of HIFU for noninvasive ablation were reported in the early 1940 s n Animal studies

High Intensity Focused Ultrasound n High intensity q n 1000 W/cm 2 to 25000 W/cm 2 Focus

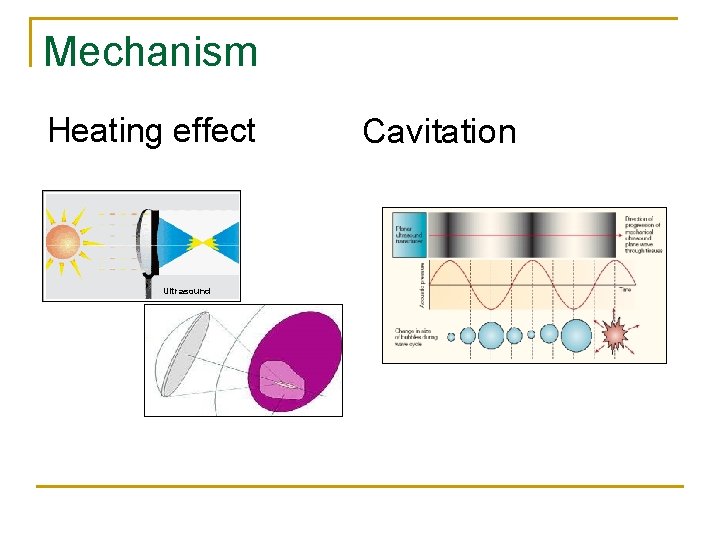
Mechanism Heating effect Ultrasound Cavitation

‘Lesion’ of coagulation necrosis at focus Target organ (e. g. liver) Transducer Tumor Skin Undamaged tissue surrounding focus

HIFU at Queen Mary Hospital HIFU service for HCC started in Oct 2006 thanks to donation of the USG-guided HIFU system by Chongqing Haifu Co.

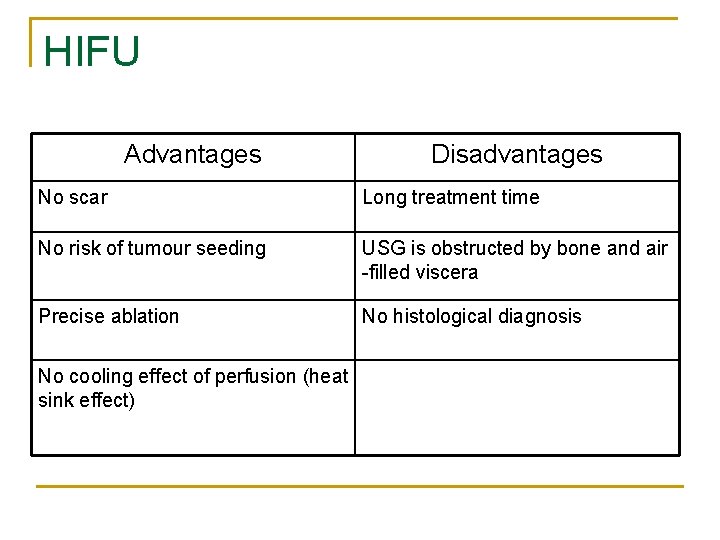
HIFU Advantages Disadvantages No scar Long treatment time No risk of tumour seeding USG is obstructed by bone and air -filled viscera Precise ablation No histological diagnosis No cooling effect of perfusion (heat sink effect)

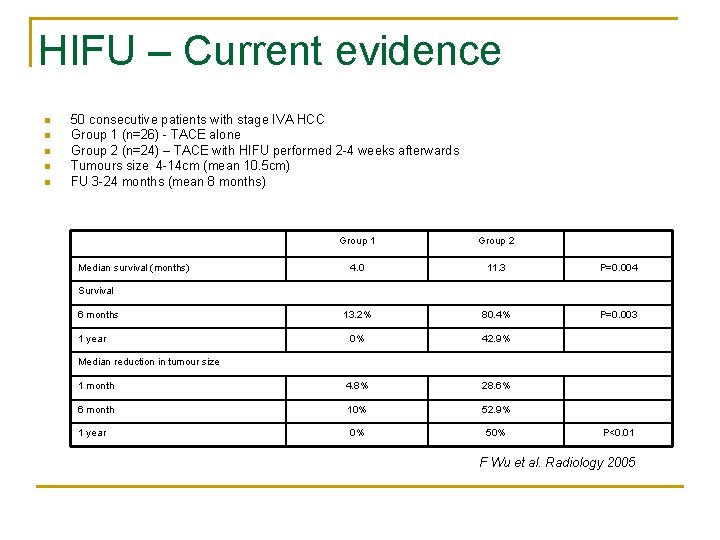
HIFU – Current evidence n n n 50 consecutive patients with stage IVA HCC Group 1 (n=26) - TACE alone Group 2 (n=24) – TACE with HIFU performed 2 -4 weeks afterwards Tumours size 4 -14 cm (mean 10. 5 cm) FU 3 -24 months (mean 8 months) Group 1 Group 2 4. 0 11. 3 P=0. 004 13. 2% 80. 4% P=0. 003 0% 42. 9% 1 month 4. 8% 28. 6% 6 month 10% 52. 9% 1 year 0% 50% Median survival (months) Survival 6 months 1 year Median reduction in tumour size P<0. 01 F Wu et al. Radiology 2005

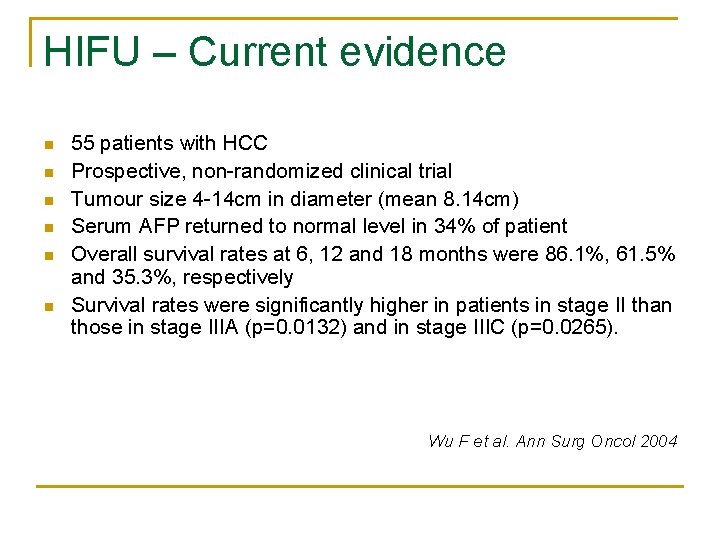
HIFU – Current evidence n n n 55 patients with HCC Prospective, non-randomized clinical trial Tumour size 4 -14 cm in diameter (mean 8. 14 cm) Serum AFP returned to normal level in 34% of patient Overall survival rates at 6, 12 and 18 months were 86. 1%, 61. 5% and 35. 3%, respectively Survival rates were significantly higher in patients in stage II than those in stage IIIA (p=0. 0132) and in stage IIIC (p=0. 0265). Wu F et al. Ann Surg Oncol 2004

HIFU at Queen Mary Hospital n From October 2006 to April 2008, totally 41 patients with unresectable HCC received HIFU treatment n Initial 21 patients received HIFU with transarterial embolization (TAE) (Period 1), and subsequent 20 patients received HIFU without TAE (Period 2)

Demographic and Clinical Data Age, years Sex ratio, M: F Hepatitis B surface antigen positive Hepatitis C virus antibody positive Period 1 (n=21) Period 2 (n=20) P-value 65 (48 – 84) 68 (55 – 77) 0. 271 17: 4 17: 3 1. 000 14 (66. 7%) 17 (85%) 0. 277 4 (19%) 3 (15%) 1. 000 Child-Pugh classification 0. 643 Class A 17 (81%) 19 (95%) Class B 4 (19%) 3 (15%) Previous hepatic resection 8 (34. 7%) 5 (38. 4%) 1. 000 Previous TACE 8 (38. 1%) 7 (35%) 0. 837 10 (2 -3951) 10 (3 -8840) 0. 766 Serum alpha fetoprotein, ng/ml

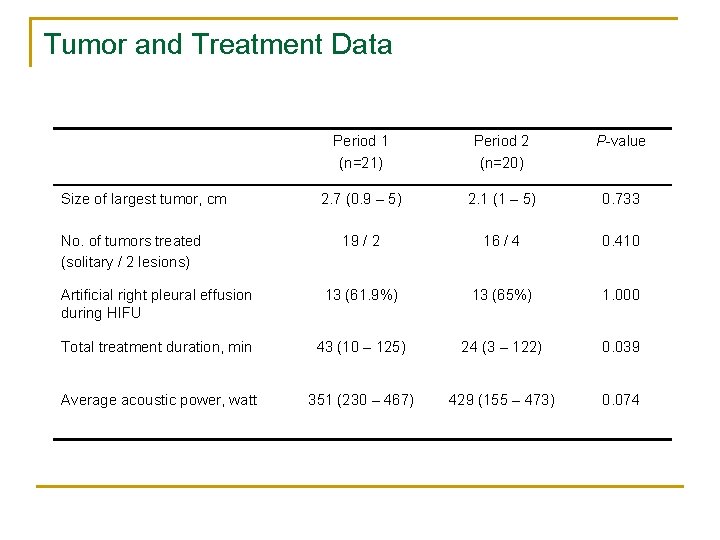
Tumor and Treatment Data Period 1 (n=21) Period 2 (n=20) P-value 2. 7 (0. 9 – 5) 2. 1 (1 – 5) 0. 733 19 / 2 16 / 4 0. 410 Artificial right pleural effusion during HIFU 13 (61. 9%) 13 (65%) 1. 000 Total treatment duration, min 43 (10 – 125) 24 (3 – 122) 0. 039 Average acoustic power, watt 351 (230 – 467) 429 (155 – 473) 0. 074 Size of largest tumor, cm No. of tumors treated (solitary / 2 lesions)

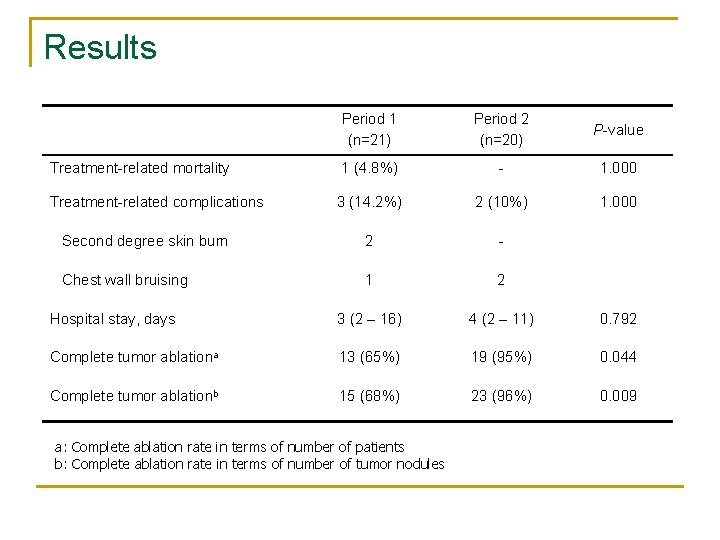
Results Period 1 (n=21) Period 2 (n=20) P-value Treatment-related mortality 1 (4. 8%) - 1. 000 Treatment-related complications 3 (14. 2%) 2 (10%) 1. 000 Second degree skin burn 2 - Chest wall bruising 1 2 Hospital stay, days 3 (2 – 16) 4 (2 – 11) 0. 792 Complete tumor ablationa 13 (65%) 19 (95%) 0. 044 Complete tumor ablationb 15 (68%) 23 (96%) 0. 009 a: Complete ablation rate in terms of number of patients b: Complete ablation rate in terms of number of tumor nodules

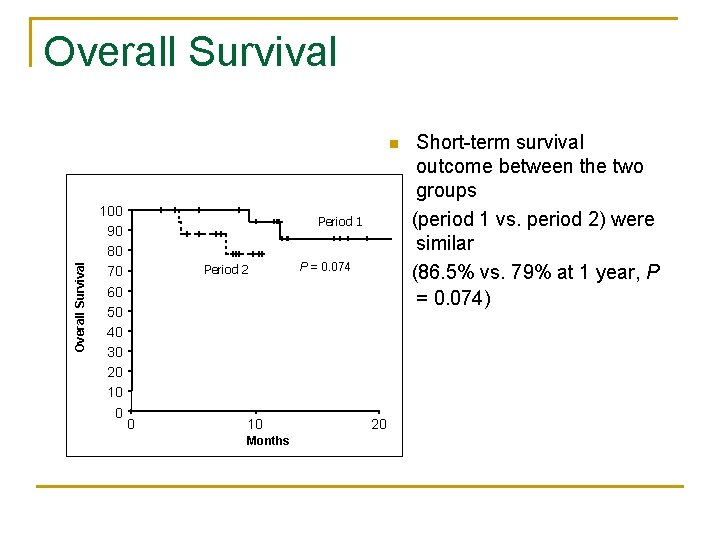
Overall Survival n 100 90 80 70 60 50 40 30 20 10 0 Period 1 Period 2 P = 0. 074 Short-term survival outcome between the two groups (period 1 vs. period 2) were similar (86. 5% vs. 79% at 1 year, P = 0. 074) Period 1 Period 2 0 10 Months 20

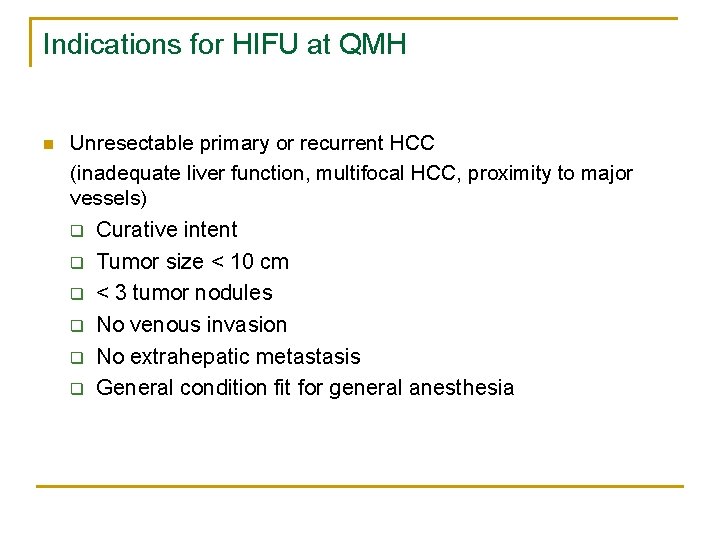
Indications for HIFU at QMH n Unresectable primary or recurrent HCC (inadequate liver function, multifocal HCC, proximity to major vessels) q Curative intent q q q Tumor size < 10 cm < 3 tumor nodules No venous invasion No extrahepatic metastasis General condition fit for general anesthesia

Discussion n n Its application as a non-invasive surgical tool is still in its infancy Limitations and challenges Indications Limited evidence
 Ferriman–gallwey score
Ferriman–gallwey score Osteochondroma gross pathology
Osteochondroma gross pathology Adrenal tumour
Adrenal tumour Mobile phone brain tumour
Mobile phone brain tumour Yanina debenedetti
Yanina debenedetti Hifu
Hifu High intensity flag football
High intensity flag football Sim high intensity network
Sim high intensity network High intensity x-ray beam machine
High intensity x-ray beam machine Fspos
Fspos Typiska novell drag
Typiska novell drag Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Vad står k.r.å.k.a.n för
Vad står k.r.å.k.a.n för Varför kallas perioden 1918-1939 för mellankrigstiden
Varför kallas perioden 1918-1939 för mellankrigstiden En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Kassaregister ideell förening
Kassaregister ideell förening Tidbok
Tidbok Anatomi organ reproduksi
Anatomi organ reproduksi Vad är densitet
Vad är densitet