Hetero cyclic Analogues of Naphthalene with one heteroatom


- Slides: 20

Hetero cyclic Analogues of Naphthalene with one heteroatom The most investigated compound of this group contain a nitrogen atom , and theoretically can be drived from naphthalene by the substation of carbon or carbon and hydrogen atoms by a nitrogen atom. The same result can be obtained by fusing a benzene on to that of pyridine and three structure result. Derivative of all three ring system 1 , 2 , 3 occure naturally in the form of alkaloide lec 9 1

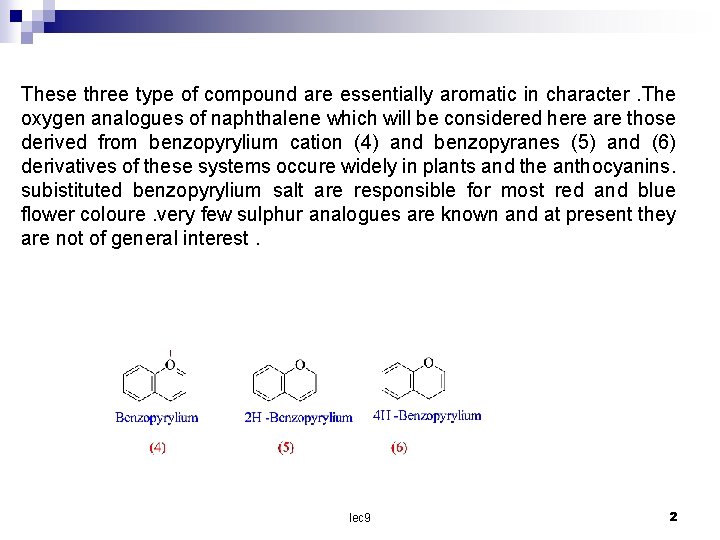
These three type of compound are essentially aromatic in character. The oxygen analogues of naphthalene which will be considered here are those derived from benzopyrylium cation (4) and benzopyranes (5) and (6) derivatives of these systems occure widely in plants and the anthocyanins. subistituted benzopyrylium salt are responsible for most red and blue flower coloure. very few sulphur analogues are known and at present they are not of general interest. lec 9 2

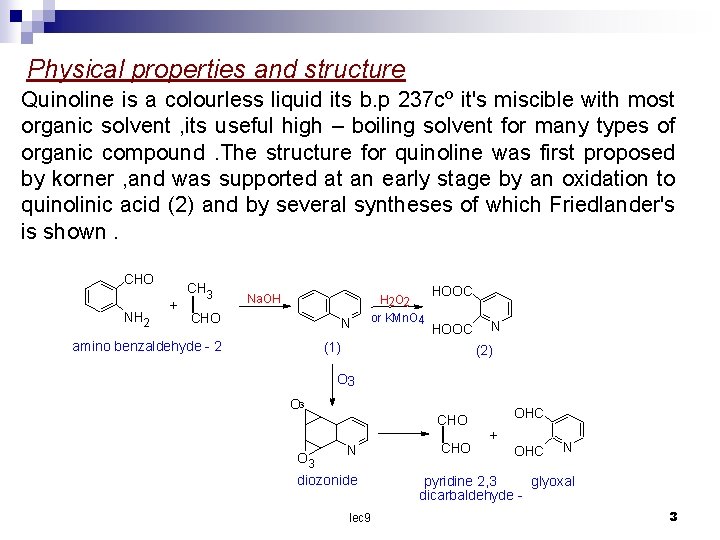
Physical properties and structure Quinoline is a colourless liquid its b. p 237 cº it's miscible with most organic solvent , its useful high – boiling solvent for many types of organic compound. The structure for quinoline was first proposed by korner , and was supported at an early stage by an oxidation to quinolinic acid (2) and by several syntheses of which Friedlander's is shown. CHO NH 2 + CH 3 | Na. OH CHO N amino benzaldehyde 2 H 2 O 2 or KMn. O 4 HOOC (1 ) N (2 ) O 3 O 3 OHC CHO N diozonide lec 9 | CHO + OHC N pyridine 2, 3 glyoxal dicarbaldehyde 3

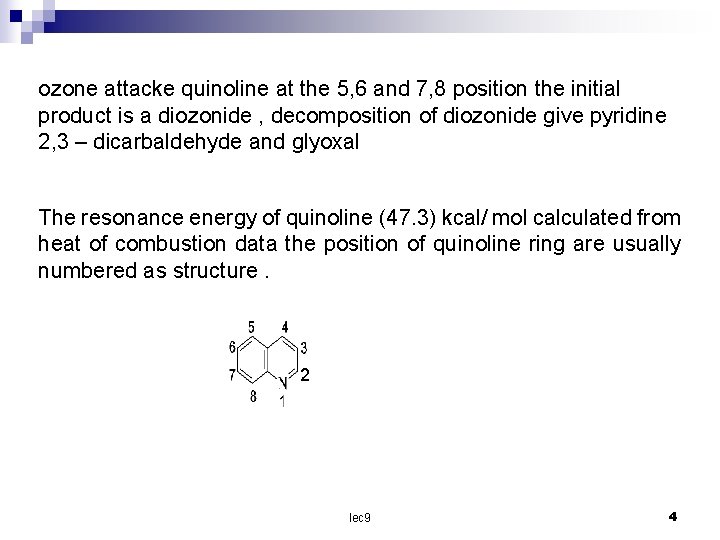
ozone attacke quinoline at the 5, 6 and 7, 8 position the initial product is a diozonide , decomposition of diozonide give pyridine 2, 3 – dicarbaldehyde and glyoxal The resonance energy of quinoline (47. 3) kcal/ mol calculated from heat of combustion data the position of quinoline ring are usually numbered as structure. 2 lec 9 4

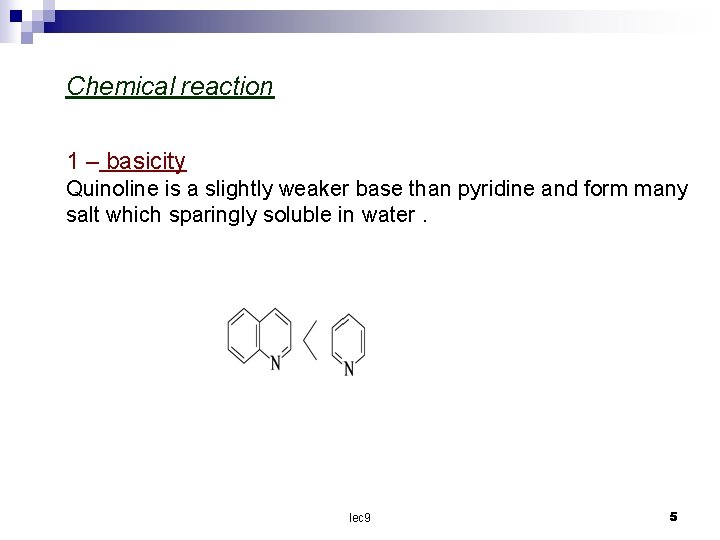
Chemical reaction 1 – basicity Quinoline is a slightly weaker base than pyridine and form many salt which sparingly soluble in water. lec 9 5

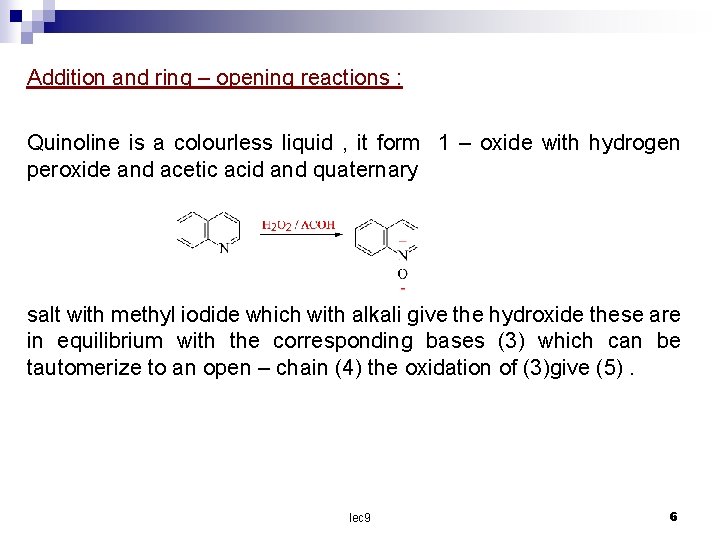
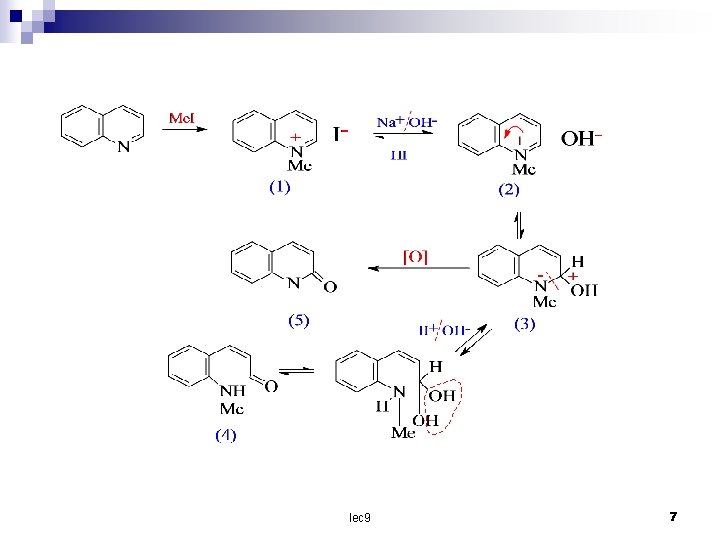
Addition and ring – opening reactions : Quinoline is a colourless liquid , it form 1 – oxide with hydrogen peroxide and acetic acid and quaternary salt with methyl iodide which with alkali give the hydroxide these are in equilibrium with the corresponding bases (3) which can be tautomerize to an open – chain (4) the oxidation of (3)give (5). lec 9 6

lec 9 7

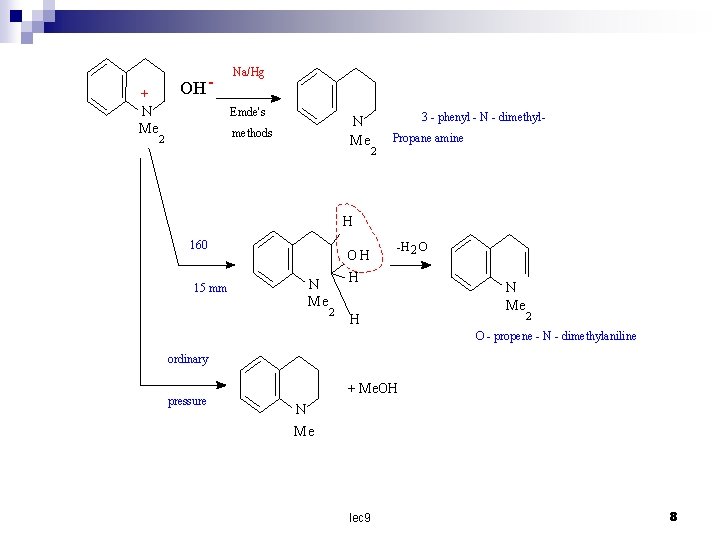
+ N Me OH - Na/Hg Emde's N Me methods 2 3 - phenyl - N - dimethyl. Propane amine 2 H 160 OH N Me 15 mm -H 2 O H 2 H N Me 2 O - propene - N - dimethylaniline ordinary pressure + Me. OH N Me lec 9 8

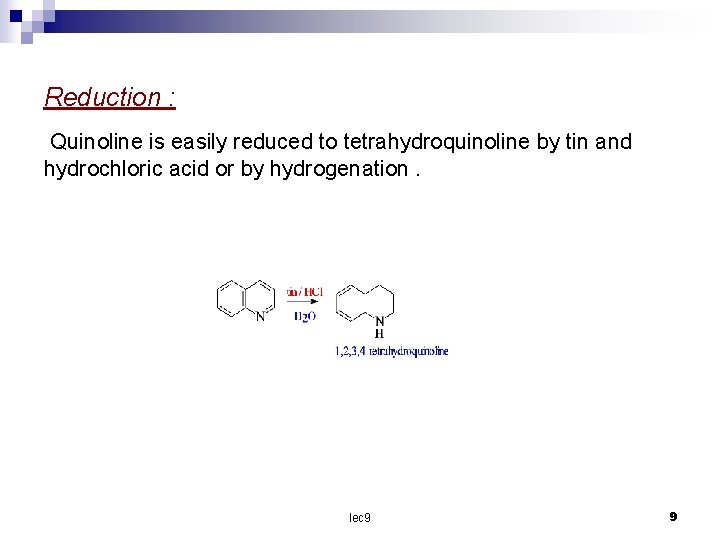
Reduction : Quinoline is easily reduced to tetrahydroquinoline by tin and hydrochloric acid or by hydrogenation. lec 9 9

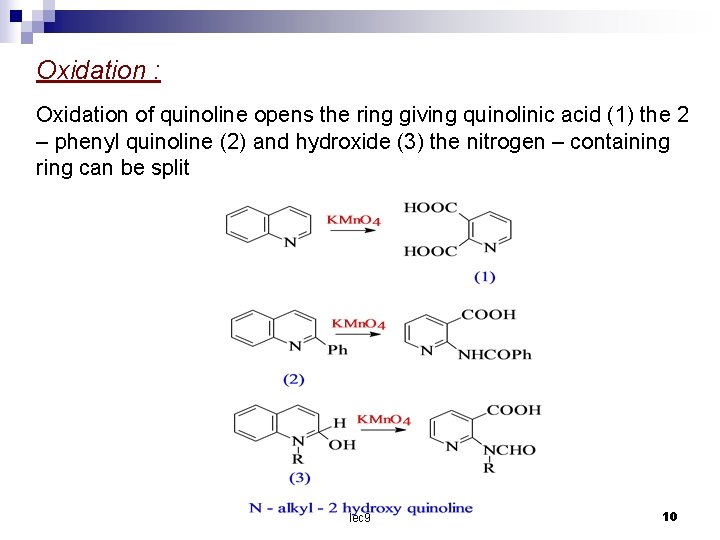
Oxidation : Oxidation of quinoline opens the ring giving quinolinic acid (1) the 2 – phenyl quinoline (2) and hydroxide (3) the nitrogen – containing ring can be split lec 9 10

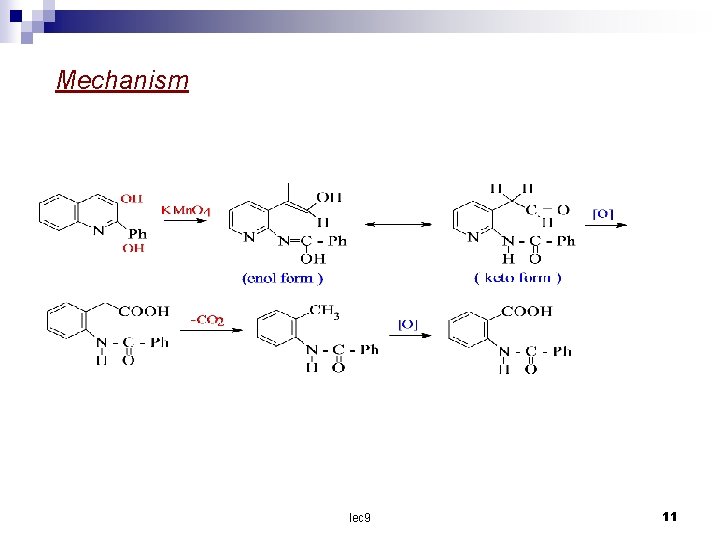
Mechanism lec 9 11

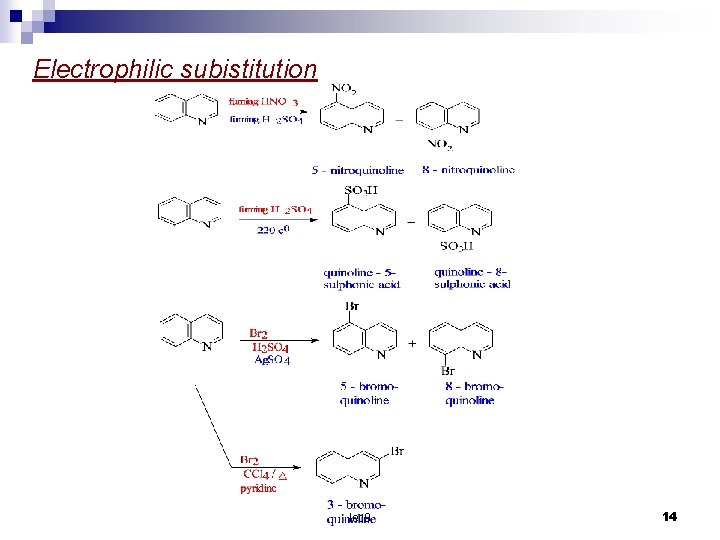
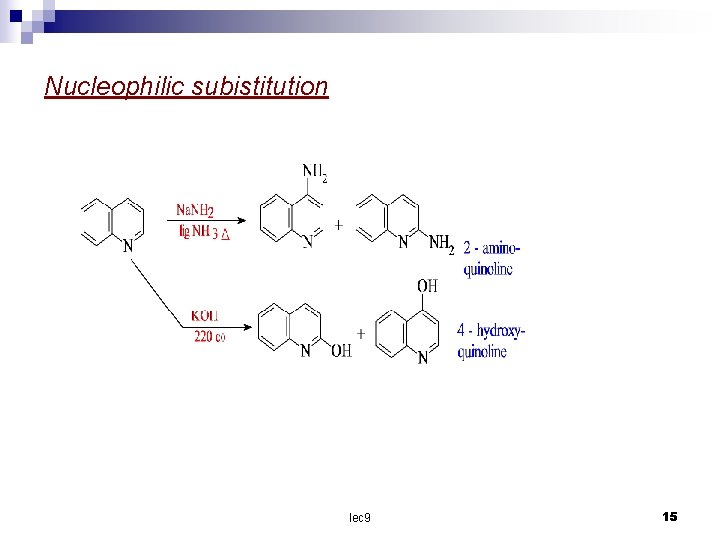
Substitution reaction The nitrogen atom deactivates the pyridine ring and so the subistituent enter the benzene ring. calculation of charge densities show that position 8 will be attack by electrophilic reagent and position 2 by nucleophilic reagents. In practice nitration and sulphonation give a mixture of – 5 and 8 – substituted product. In bromination the heterocyclic rings is subistituted in the 3 – position. lec 9 12

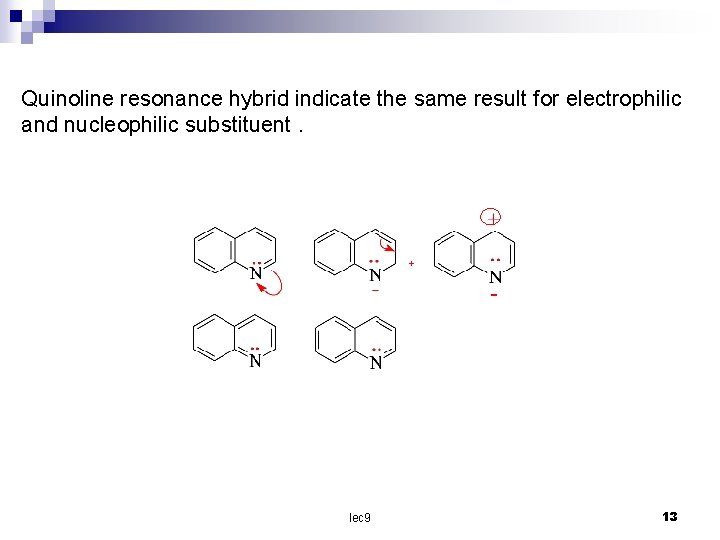
Quinoline resonance hybrid indicate the same result for electrophilic and nucleophilic substituent. + lec 9 13

Electrophilic subistitution lec 9 14

Nucleophilic subistitution lec 9 15

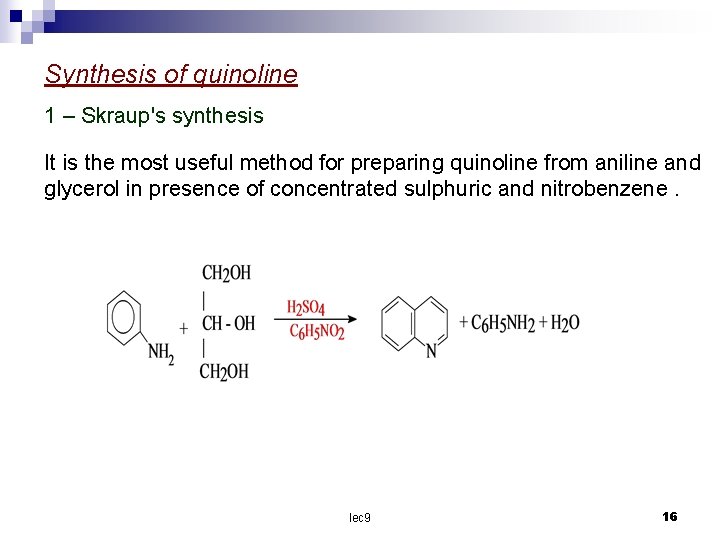
Synthesis of quinoline 1 – Skraup's synthesis It is the most useful method for preparing quinoline from aniline and glycerol in presence of concentrated sulphuric and nitrobenzene. lec 9 16

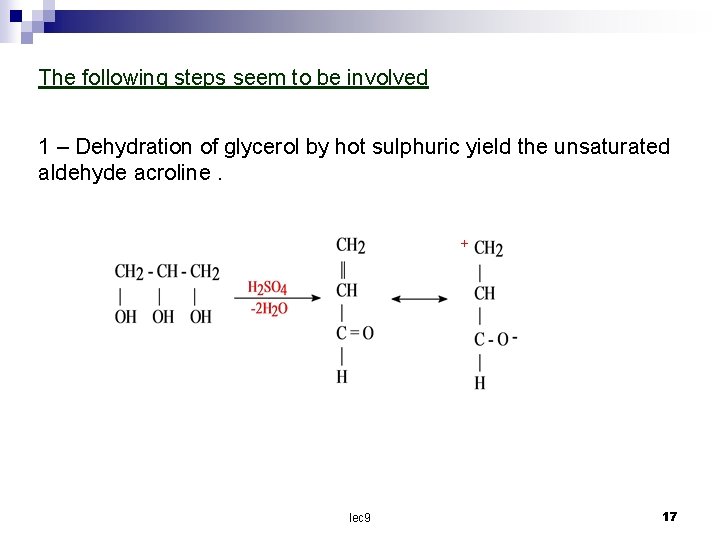
The following steps seem to be involved 1 – Dehydration of glycerol by hot sulphuric yield the unsaturated aldehyde acroline. + lec 9 17

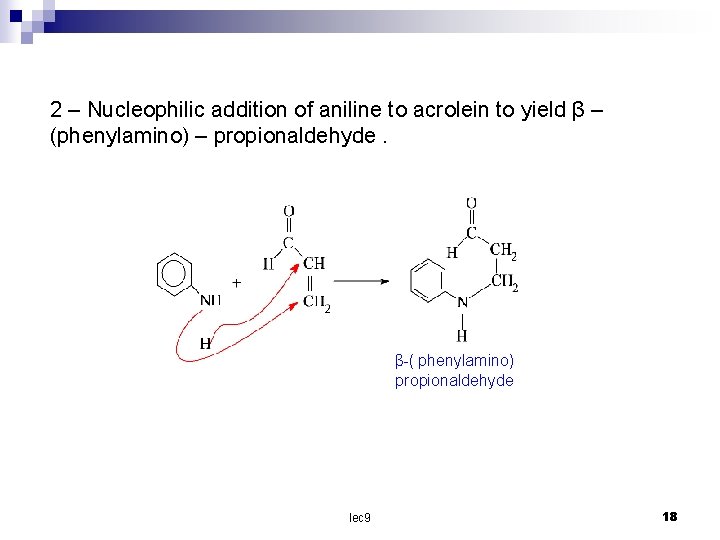
2 – Nucleophilic addition of aniline to acrolein to yield β – (phenylamino) – propionaldehyde. β ( phenylamino) propionaldehyde lec 9 18

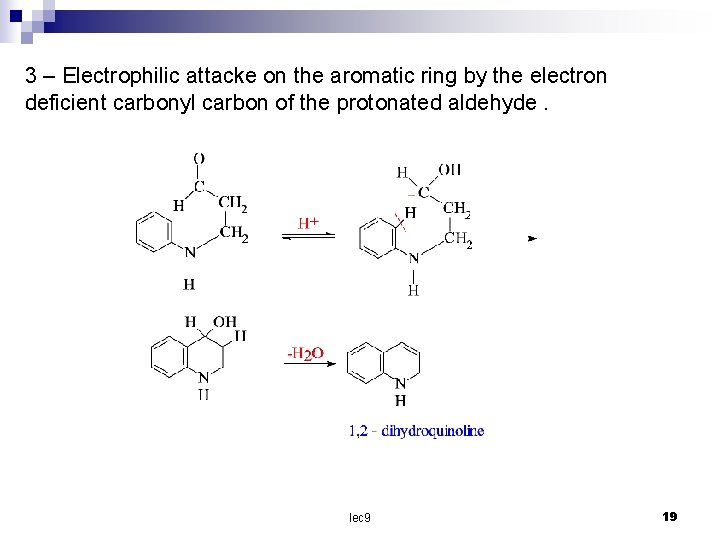
3 – Electrophilic attacke on the aromatic ring by the electron deficient carbonyl carbon of the protonated aldehyde. lec 9 19

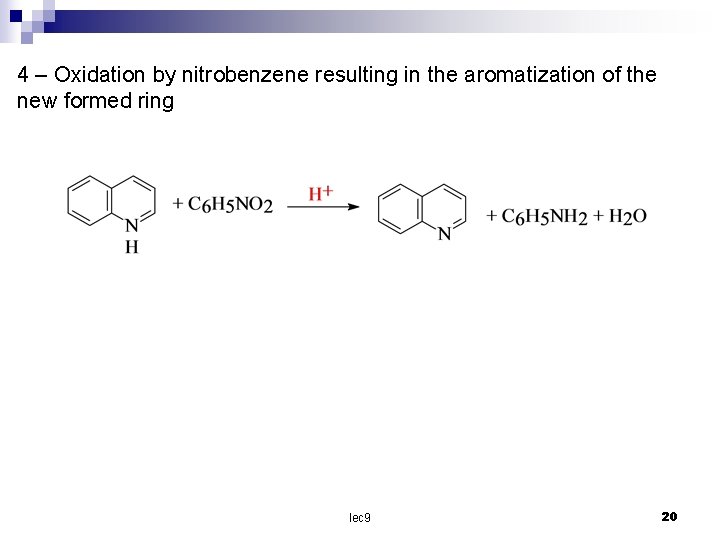
4 – Oxidation by nitrobenzene resulting in the aromatization of the new formed ring lec 9 20
 Heteroatom examples
Heteroatom examples Hetero sugesti adalah
Hetero sugesti adalah Hetero atom
Hetero atom Holozoik beslenme
Holozoik beslenme Heteroanamnesis
Heteroanamnesis Hetero hypno
Hetero hypno Hetero medical terminology
Hetero medical terminology Hétéro sondage
Hétéro sondage Linguo incisal ridge
Linguo incisal ridge Hoch2oh boiling point
Hoch2oh boiling point Posher synthesis
Posher synthesis Fittig synthesis of naphthalene
Fittig synthesis of naphthalene Naphthalene chemical name
Naphthalene chemical name Studiendekanat uni bonn
Studiendekanat uni bonn One empire one god one emperor
One empire one god one emperor See one do one teach one
See one do one teach one One vision one identity one community
One vision one identity one community One king one law one faith
One king one law one faith One one little dog run
One one little dog run One price policy
One price policy Asean one vision one identity one community
Asean one vision one identity one community