Harris Chapter 13 Ethylene Diamine Tetra Acetic acid


- Slides: 8

Harris Chapter 13 Ethylene. Diamine. Tetra. Acetic acid Chapter 16 starts with slide 6

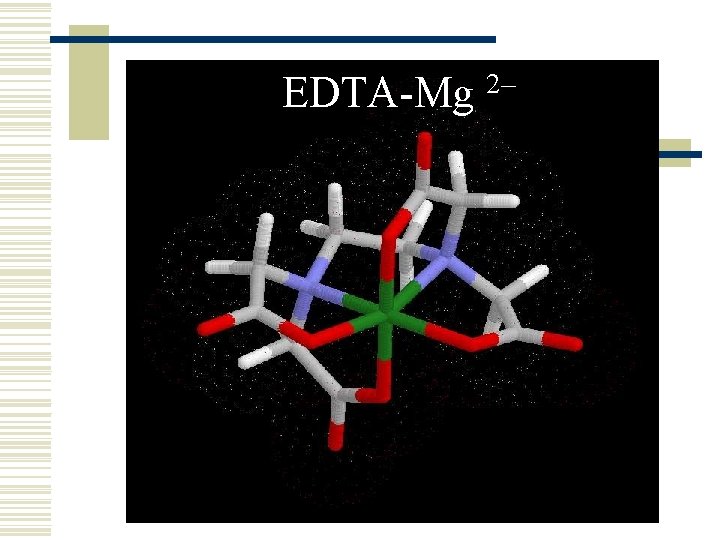
EDTA-Mg 2–

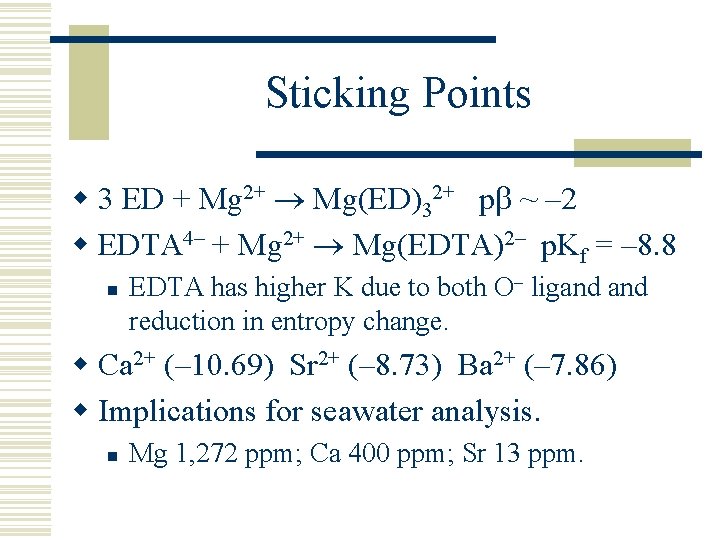
Sticking Points w 3 ED + Mg 2+ Mg(ED)32+ p ~ – 2 w EDTA 4– + Mg 2+ Mg(EDTA)2– p. Kf = – 8. 8 n EDTA has higher K due to both O– ligand reduction in entropy change. w Ca 2+ (– 10. 69) Sr 2+ (– 8. 73) Ba 2+ (– 7. 86) w Implications for seawater analysis. n Mg 1, 272 ppm; Ca 400 ppm; Sr 13 ppm.

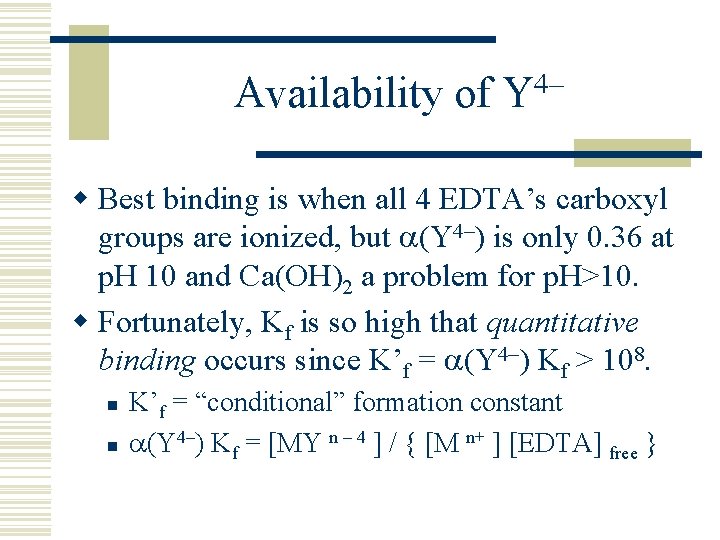
Availability of Y 4– w Best binding is when all 4 EDTA’s carboxyl groups are ionized, but (Y 4–) is only 0. 36 at p. H 10 and Ca(OH)2 a problem for p. H>10. w Fortunately, Kf is so high that quantitative binding occurs since K’f = (Y 4–) Kf > 108. n n K’f = “conditional” formation constant (Y 4–) Kf = [MY n – 4 ] / { [M n+ ] [EDTA] free }

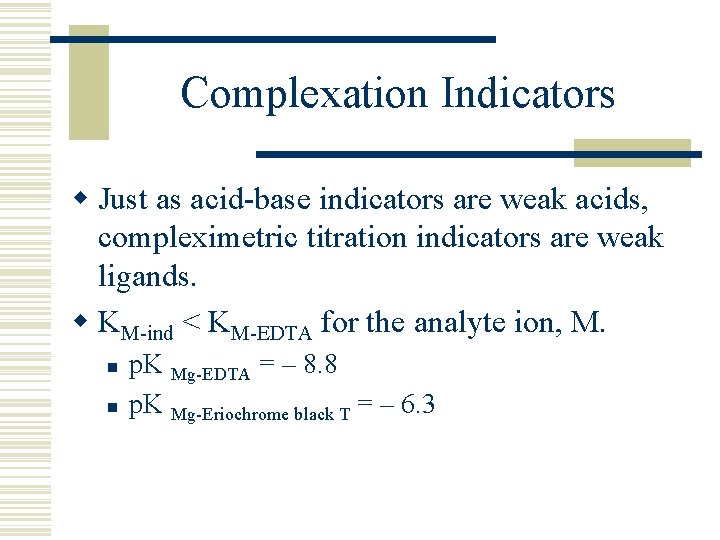
Complexation Indicators w Just as acid-base indicators are weak acids, compleximetric titration indicators are weak ligands. w KM-ind < KM-EDTA for the analyte ion, M. n n p. K Mg-EDTA = – 8. 8 p. K Mg-Eriochrome black T = – 6. 3

Harris Chapter 16 Redox Titrations

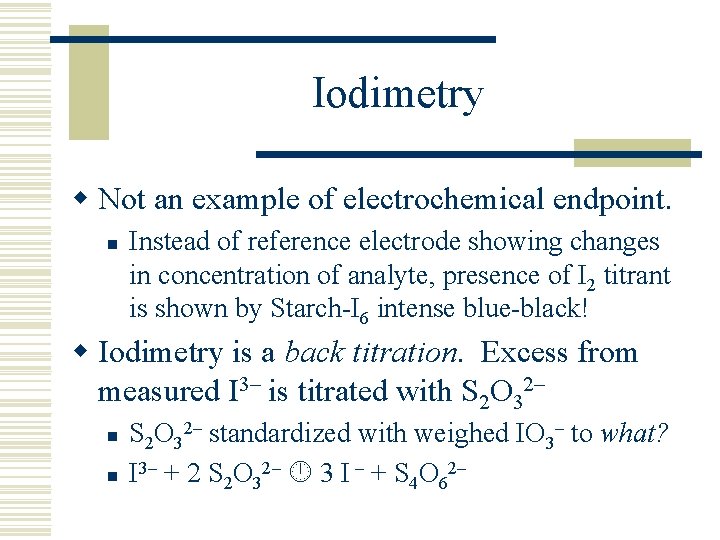
Iodimetry w Not an example of electrochemical endpoint. n Instead of reference electrode showing changes in concentration of analyte, presence of I 2 titrant is shown by Starch-I 6 intense blue-black! w Iodimetry is a back titration. Excess from measured I 3– is titrated with S 2 O 32– n n S 2 O 32– standardized with weighed IO 3– to what? I 3– + 2 S 2 O 32– 3 I – + S 4 O 62–

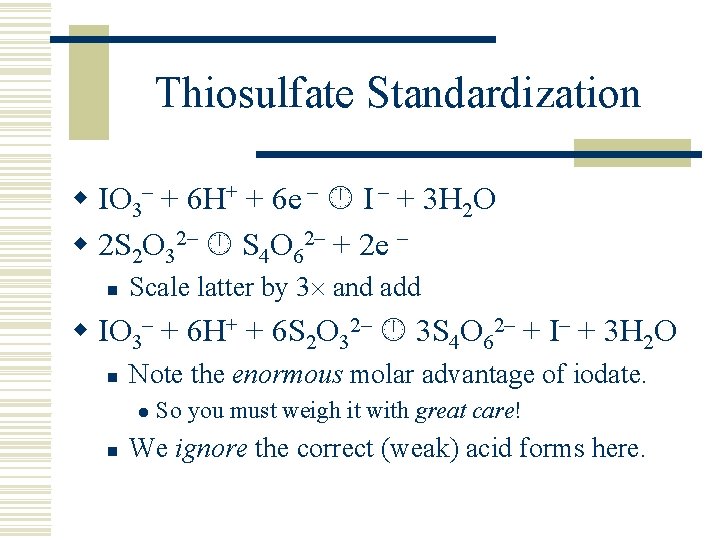
Thiosulfate Standardization w IO 3– + 6 H+ + 6 e – I – + 3 H 2 O w 2 S 2 O 32– S 4 O 62– + 2 e – n Scale latter by 3 and add w IO 3– + 6 H+ + 6 S 2 O 32– 3 S 4 O 62– + I– + 3 H 2 O n Note the enormous molar advantage of iodate. l n So you must weigh it with great care! We ignore the correct (weak) acid forms here.