Hair Dyes Hair dyes fall naturally in three







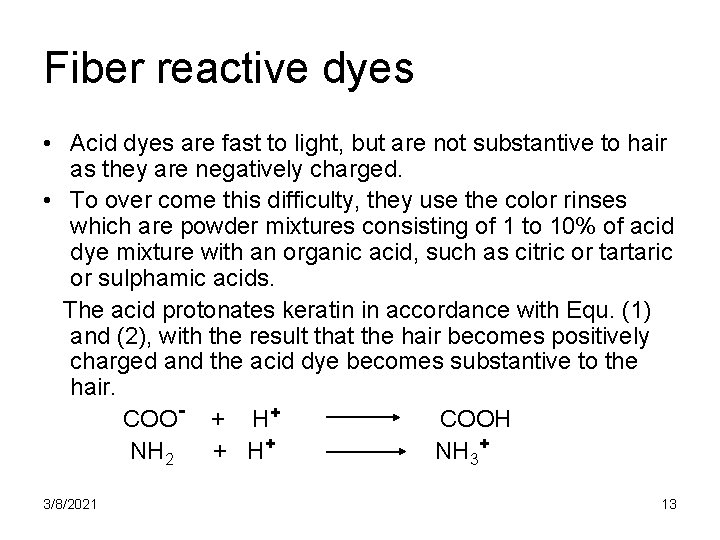








- Slides: 24

Hair Dyes Hair dyes fall naturally in three classes: 1 - Temporary 2 - Permanent 3 - Semi-permanent 3/8/2021 1

Temporary Dyes • Which last, at the most, until the next shampoo • These take the form of a modified setting lotion. • A suitable dye or mixture of dyes is added to a solution of a film former, on drying a colored transparent envelope is left around each hair fiber. 3/8/2021 2

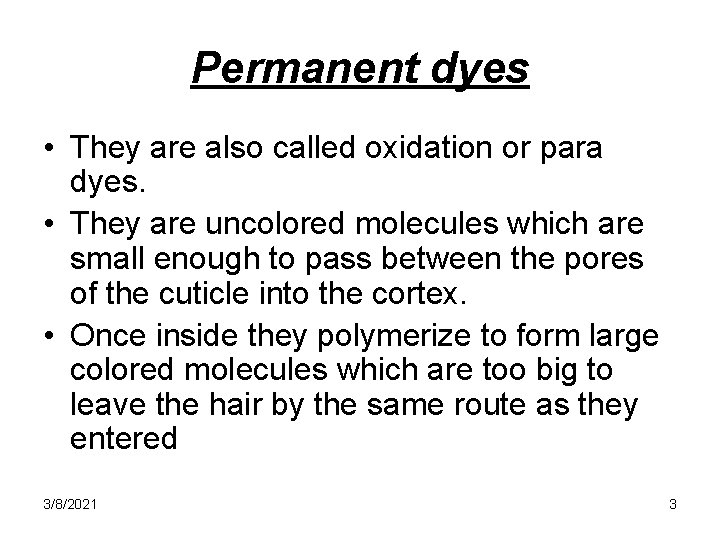
Permanent dyes • They are also called oxidation or para dyes. • They are uncolored molecules which are small enough to pass between the pores of the cuticle into the cortex. • Once inside they polymerize to form large colored molecules which are too big to leave the hair by the same route as they entered 3/8/2021 3

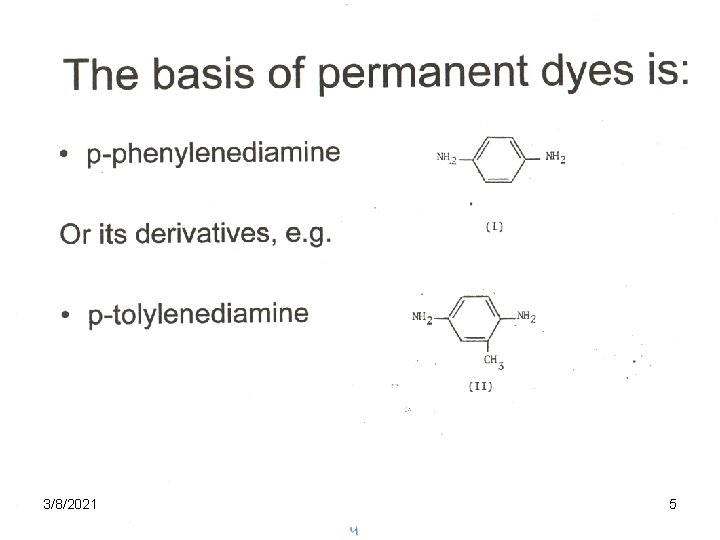
The basis of permanent dyes is: • p-phenylenediamine Or its derivatives, e. g. • p-tolylenediamine 3/8/2021 4

3/8/2021 5

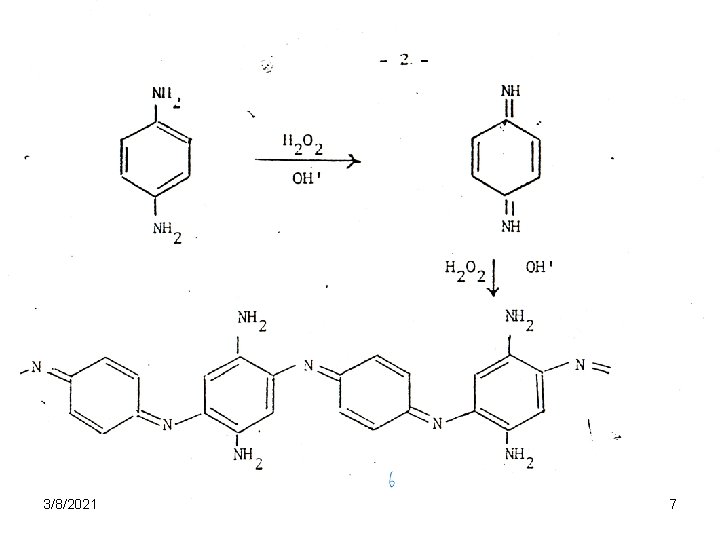
Permanent dyes • These molecules are small enough to enter the cortex when applied in alkaline solution, which swells the hair and provides easier access. • The dye mixture, dissolved in ammonia solution is mixed with hydrogen peroxide immediately before application. • The diamines penetrate the hair, where they are oxidized to form quinone, which polymerizes (Eq. 1) 3/8/2021 6

3/8/2021 7

Permanent dyes • The polymer is a long chain of alternating double and single bonds, and is therefore dark brown to black in color. • The color of the polymer is varied by complexing other compounds with it. • Thus catechol (III) gives gray color, • 2 -nitro-1, 4 phenylenediamine (IV), brownish red, and so on. 3/8/2021 8

3/8/2021 9

Semi-permanent dyes • They vary in efficiency from a very good temporary to a poor permanent. • They have moderate resistance to shampooing. • Hydrogen peroxide is not used in their devlopment. 3/8/2021 10

Semi-permanent dyes are classified as follows: 1 - Fiber reactive dyes These become fixed to the hair through chemical groups on the dye molecule, which interact with keratin. - Basic dyes : have positive charge on the active part of the molecule. Methylene blue is an example, they are substantive to hair and, unfortunately to the scalp. 3/8/2021 11
Fiber reactive dyes • Basic dyes have the disadvantage that they are not fast to light. - Acid dyes : have negative charge which usually comes from a sulphonic acid group as with orange G (VI), but some time carboxy group is involved. 3/8/2021 12
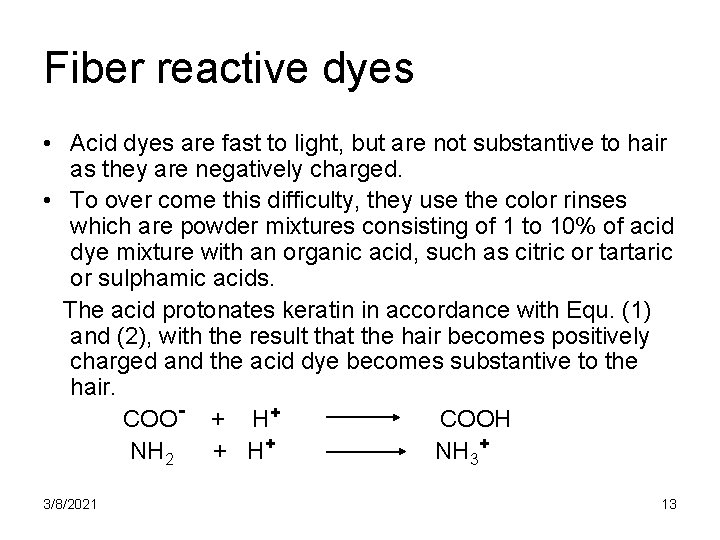
Fiber reactive dyes • Acid dyes are fast to light, but are not substantive to hair as they are negatively charged. • To over come this difficulty, they use the color rinses which are powder mixtures consisting of 1 to 10% of acid dye mixture with an organic acid, such as citric or tartaric or sulphamic acids. The acid protonates keratin in accordance with Equ. (1) and (2), with the result that the hair becomes positively charged and the acid dye becomes substantive to the hair. COO- + H+ COOH NH 2 + H+ NH 3+ 3/8/2021 13

Acid dyes • A more recent trend is to synthesize dyes which contain at least one quaternary ammonium group. - Basic brown 16 (VII) is an example. It gives a mahogany color and does not fade. 3/8/2021 14

2 - Oxidation dyes These are so called because the dye molecules are similar to those used in permanent oxidation dyes a- Nitro dyes - are small, colored molecules which pentrate the cuticle and remain in the cortex. Because they are small molecules, they are gradually leached out with subsequent shampooings - Examples are 2 - nitro-1, 4 -phenylenediamine (red) and 4 - nitro-1, 2 -phenylenediamine (yellow) 3/8/2021 15

2 - Oxidation dyes b- Dyes developed by atmospheric oxidation - are colorless molecules which develop a color on contact with the atmosphere. Example – 2, 4, 6 - Triaminophenol. It is applied as 3% solution at p. H 8 and the color develops in about 15 minutes. 3/8/2021 16

3 - Solvent assisted dyes • In the textile industry, dyes are applied to wool from a hot nearly boiling dye bath. • This is obviously too hot for human hair. • Temperature is also a problem in textile industry, because wool shrinks at high temperatures. • This has been overcome by adding an organic solvent to the dye aqueous solution. 3/8/2021 17

3 - Solvent assisted dyes • The solvent must be only sparingly soluble in water, and good solvent for the dye, but not as good as the hair. The aqueous phase then acts as a reservoir from which the dye is transferred continuously to the fiber. • Benzyl alcohol (soluble 1 in 400 in water) is the most popular solvent. • An electrolyte, e. g. sodium sulphate or formic acid are added to reduce solubility of acid dye. 3/8/2021 18

4 - Disperse dyes • Are water insoluble dyes which are applied to the hair as an aqueous suspension. • The dyes are hydrophobic, and as such are substantive to keratin 5 - Metallic dyes The most commonly used materials for this method are lead acetate. A suspension of precipitated sulfur in lead acetate solution, applied daily, will slowly deposit a mixture of lead sulfide and lead oxide on the cuticle, so that the hair slowly darkens. 3/8/2021 19

6 - Vegetable dyes • • • Henna (lawsonia inermis) Walnut (Juglas regia) Commomile Hibiscus Senna (Neutral henna) 3/8/2021 20

A quantitative study of the effect of p. H on the dyeing process with juglone B. I. AMRO, Faculty of Pharmacy, University of Jordan Visiting Associate Prof. , Faculty of Pharmacy, Amman University • Bleached wool felts have been dyed with juglone, the principal coloring agent of walnut. • A dissolution technique that could evaluate uptake and substantivity was adopted. The mass of juglone removed from the wool was assessed from the reflectance readings related to changes in L. a. b. values of dyed felts which were recorded before and after extraction. • A theory linking dyeing performance to electrolytic dissociation of juglone and keratin protonation was developed from the results. 3/8/2021 21

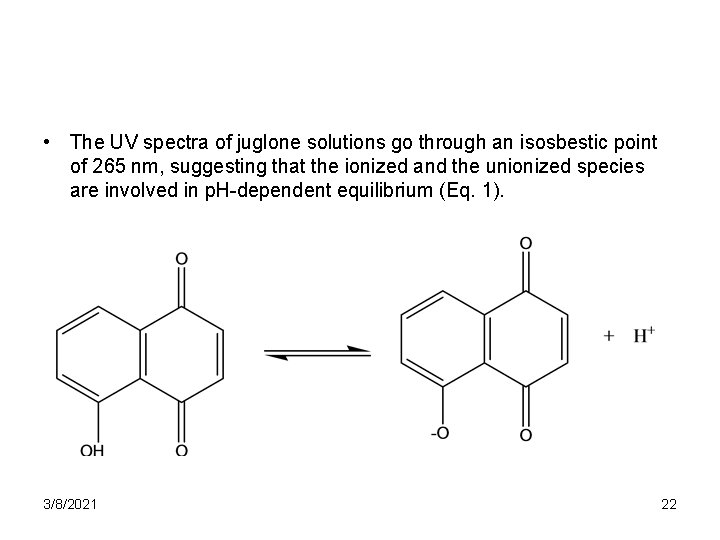
• The UV spectra of juglone solutions go through an isosbestic point of 265 nm, suggesting that the ionized and the unionized species are involved in p. H-dependent equilibrium (Eq. 1). 3/8/2021 22

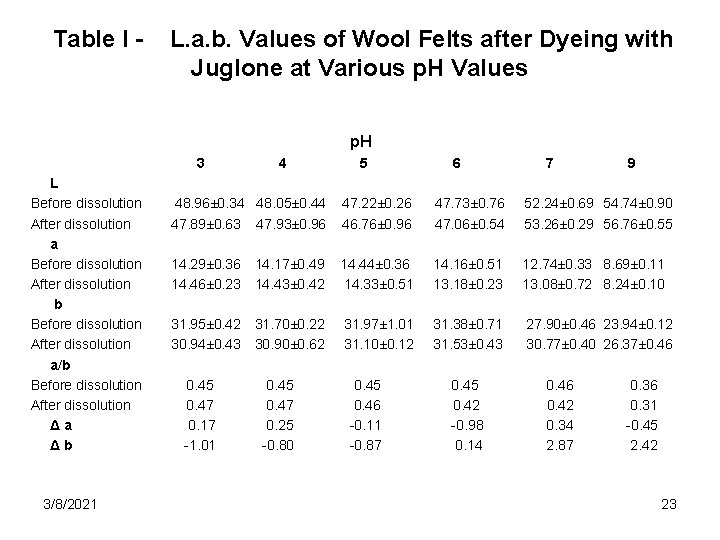
Table I - L. a. b. Values of Wool Felts after Dyeing with Juglone at Various p. H Values p. H 3 L Before dissolution After dissolution a Before dissolution After dissolution b Before dissolution After dissolution a/b Before dissolution After dissolution Δa Δb 3/8/2021 4 5 6 7 9 48. 96± 0. 34 48. 05± 0. 44 47. 89± 0. 63 47. 93± 0. 96 47. 22± 0. 26 46. 76± 0. 96 47. 73± 0. 76 47. 06± 0. 54 52. 24± 0. 69 54. 74± 0. 90 53. 26± 0. 29 56. 76± 0. 55 14. 29± 0. 36 14. 17± 0. 49 14. 46± 0. 23 14. 43± 0. 42 14. 44± 0. 36 14. 33± 0. 51 14. 16± 0. 51 13. 18± 0. 23 12. 74± 0. 33 8. 69± 0. 11 13. 08± 0. 72 8. 24± 0. 10 31. 95± 0. 42 31. 70± 0. 22 30. 94± 0. 43 30. 90± 0. 62 31. 97± 1. 01 31. 10± 0. 12 31. 38± 0. 71 31. 53± 0. 43 27. 90± 0. 46 23. 94± 0. 12 30. 77± 0. 40 26. 37± 0. 46 0. 45 0. 47 0. 17 -1. 01 0. 45 0. 47 0. 25 -0. 80 0. 45 0. 46 -0. 11 -0. 87 0. 45 0. 42 -0. 98 0. 14 0. 46 0. 42 0. 34 2. 87 0. 36 0. 31 -0. 45 2. 42 23

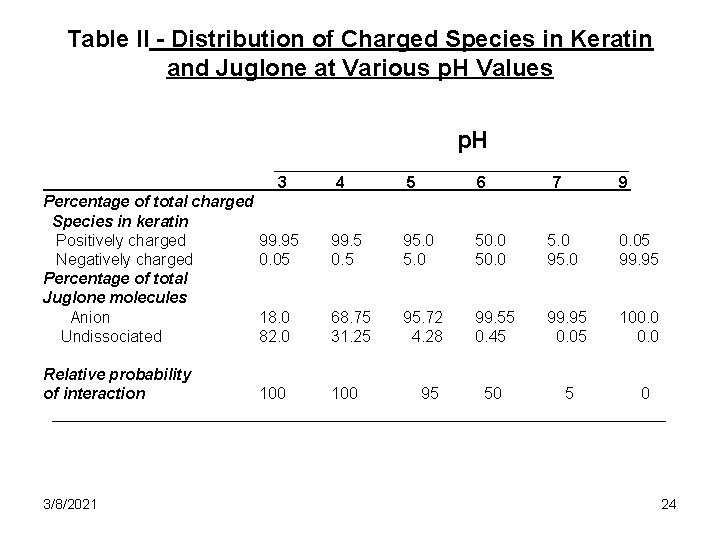
Table II - Distribution of Charged Species in Keratin and Juglone at Various p. H Values p. H ____________________ 3 4 5 6 7 9 Percentage of total charged Species in keratin Positively charged Negatively charged Percentage of total Juglone molecules Anion Undissociated 99. 95 0. 05 99. 5 0. 5 95. 0 50. 0 5. 0 95. 0 0. 05 99. 95 18. 0 82. 0 68. 75 31. 25 95. 72 4. 28 99. 55 0. 45 99. 95 0. 05 100. 0 Relative probability of interaction 100 95 50 5 0 ___________________________________ 3/8/2021 24
 Reduction of vat dyes
Reduction of vat dyes Classification of dyes on the basis of structure
Classification of dyes on the basis of structure Cambodia natural dyes
Cambodia natural dyes Paper chromatography of food dyes
Paper chromatography of food dyes Paper chromatography separation of cations and dyes
Paper chromatography separation of cations and dyes Acidic and basic dye
Acidic and basic dye Dark hair and blonde hair parents
Dark hair and blonde hair parents Tacheometric distance equation on flat and sloped terrains
Tacheometric distance equation on flat and sloped terrains How does the rebel like to have his hair
How does the rebel like to have his hair Hair grows in diagonal tubes called hair
Hair grows in diagonal tubes called hair Fibers derived entirely from animal or plant sources
Fibers derived entirely from animal or plant sources Heterozygous short-hair x heterozygous short-hair
Heterozygous short-hair x heterozygous short-hair Hair follicle histology
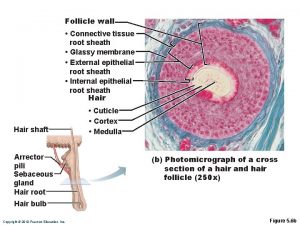
Hair follicle histology Hirsuities
Hirsuities The three layers of hair
The three layers of hair Placed half off the base of the curl.
Placed half off the base of the curl. Basic hair structure
Basic hair structure Negroid hair under microscope
Negroid hair under microscope During our lives we produce three kinds of hair
During our lives we produce three kinds of hair Three zones of hair
Three zones of hair Elements of hair design
Elements of hair design Hobbits orcs
Hobbits orcs Othello act 3 summary
Othello act 3 summary In three minutes write three things you did yesterday
In three minutes write three things you did yesterday Signs have three purposes, identify the three.
Signs have three purposes, identify the three.