Goldman Sachs Leveraged Finance Healthcare Conference 2013 March






- Slides: 18

Goldman Sachs Leveraged Finance Healthcare Conference 2013 March 6, 2013 Biomet, Inc. Daniel P. Florin Senior Vice President & Chief Financial Officer

Forward-Looking and Non-GAAP Financial Measures Forward-Looking Statements This presentation contains “forward-looking statements” within the meaning of Section 27 A of the Securities Act of 1933 and Section 21 E of the Securities Exchange Act of 1934, as amended. Those statements are often indicated by the use of words such as “will, ” “intend, ” “anticipate, ” “estimate, ” “expect, ” “plan” and similar expressions. Forward-looking statements involve certain risks and uncertainties. Actual results may differ materially from those contemplated by the forward looking statements due to, among others, the following factors: the success of the Company’s principal product lines; the results of the ongoing investigation by the United States Department of Justice; the ability to successfully implement new technologies; the Company’s ability to sustain sales and earnings growth; the Company’s success in achieving timely approval or clearance of its products with domestic and foreign regulatory entities; the impact to the business as a result of compliance with federal, state and foreign governmental regulations and with the Deferred Prosecution Agreement; the impact to the business as a result of the economic downturn in both foreign and domestic markets; the impact of federal health care reform; the impact of anticipated changes in the musculoskeletal industry and the ability of the Company to react to and capitalize on those changes; the ability of the Company to successfully implement its desired organizational changes and cost-saving initiatives; the ability of the Company to successfully integrate the Trauma Acquisition; the impact to the business as a result of the Company’s significant international operations, including, among others, with respect to foreign currency fluctuations and the success of the Company’s transition of certain manufacturing operations to China; the impact of the Company’s managerial changes; the ability of the Company’s customers to receive adequate levels of reimbursement from third-party payors; the Company’s ability to maintain its existing intellectual property rights and obtain future intellectual property rights; the impact to the business as a result of cost containment efforts of group purchasing organizations; the Company’s ability to retain existing independent sales agents for its products; the impact of product liability litigation losses; and other factors set forth in the Company’s filings with the SEC, including the Company’s most recent annual report on Form 10 -K and quarterly reports on Form 10 -Q. Although the Company believes that the assumptions on which the forward-looking statements contained herein are based are reasonable, any of those assumptions could prove to be inaccurate given the inherent uncertainties as to the occurrence or non-occurrence of future events. There can be no assurance as to the accuracy of forwardlooking statements contained in this presentation. The inclusion of a forward-looking statement herein should not be regarded as a representation by the Company that the Company’s objectives will be achieved. The Company undertakes no obligation to update publicly or revise any forward-looking statements, whether as a result of new information, future events or otherwise. Accordingly, the reader is cautioned not to place undue reliance on forward-looking statements which speak only as of the date on which they were made. Non-GAAP Financial Measures This presentation uses non-GAAP financial measures, such as net sales excluding the impact of foreign currency (constant currency), free cash flow, unlevered free cash flow, Earnings Before Interest, Taxes, Depreciation and Amortization (EBITDA) as adjusted, net debt, senior secured leverage ratio, total leverage ratio, and cash equivalents (as defined by the Company’s credit agreement) as important financial measures to review and assess financial and operating performance of its principal lines of business. Reconciliations of these non-GAAP financial measures to the most directly comparable GAAP measures are included on the Biomet website at www. biomet. com The term “adjusted” or “as adjusted, ” a non-GAAP financial measure, refers to financial performance measures that exclude certain income statement line items, such as interest, taxes, depreciation or amortization, other income (expense), and/or exclude certain expenses as defined by our credit agreement, such as restructuring charges, non-cash impairment charges, integration and facilities opening costs or other business optimization expenses, new systems design and implementation costs, certain start-up costs and costs related to consolidation of facilities, certain non-cash charges, advisory fees paid to the private equity owners, certain severance charges, purchase accounting costs, stock-based compensation and payments, payments to distributors that are not in the ordinary course of business, litigation costs, and other related charges. These non-GAAP measures are not in accordance with, or an alternative for, generally accepted accounting principles in the United States. Biomet management believes that these non-GAAP measures provide useful information to investors; however, this additional non-GAAP financial information is not meant to be considered in isolation or as a substitute for financial information prepared in accordance with GAAP. 2

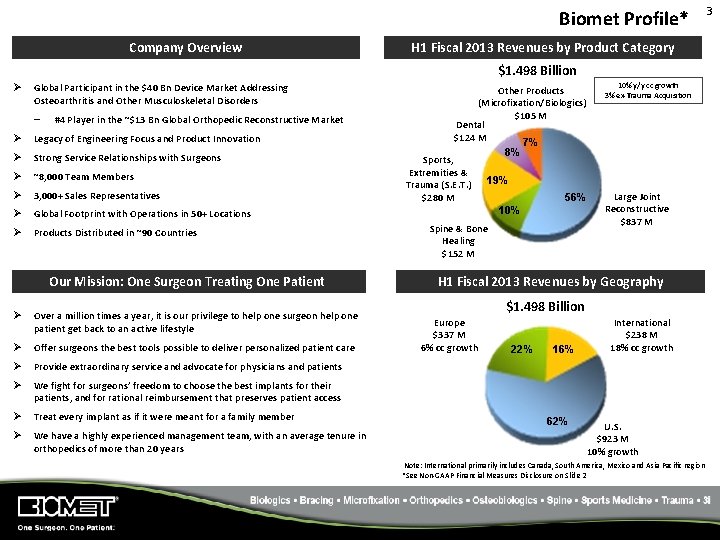
Biomet Profile* Company Overview H 1 Fiscal 2013 Revenues by Product Category $1. 498 Billion Ø Global Participant in the $40 Bn Device Market Addressing Osteoarthritis and Other Musculoskeletal Disorders – #4 Player in the ~$13 Bn Global Orthopedic Reconstructive Market Ø Legacy of Engineering Focus and Product Innovation Ø Strong Service Relationships with Surgeons Ø ~8, 000 Team Members Ø 3, 000+ Sales Representatives Ø Global Footprint with Operations in 50+ Locations Ø Products Distributed in ~90 Countries Our Mission: One Surgeon Treating One Patient Ø Over a million times a year, it is our privilege to help one surgeon help one patient get back to an active lifestyle Ø Offer surgeons the best tools possible to deliver personalized patient care Ø Provide extraordinary service and advocate for physicians and patients Ø We fight for surgeons’ freedom to choose the best implants for their patients, and for rational reimbursement that preserves patient access Ø Treat every implant as if it were meant for a family member Ø We have a highly experienced management team, with an average tenure in orthopedics of more than 20 years 10% y/y cc growth 3% ex-Trauma Acquisition Other Products (Microfixation/Biologics) $105 M Dental $124 M 7% 8% Sports, Extremities & Trauma (S. E. T. ) $280 M 19% 56% 10% Spine & Bone Healing $152 M Large Joint Reconstructive $837 M H 1 Fiscal 2013 Revenues by Geography $1. 498 Billion Europe $337 M 6% cc growth 22% 16% 62% International $238 M 18% cc growth U. S. $923 M 10% growth Note: International primarily includes Canada, South America, Mexico and Asia Pacific region *See Non‐GAAP Financial Measures Disclosure on Slide 2 3

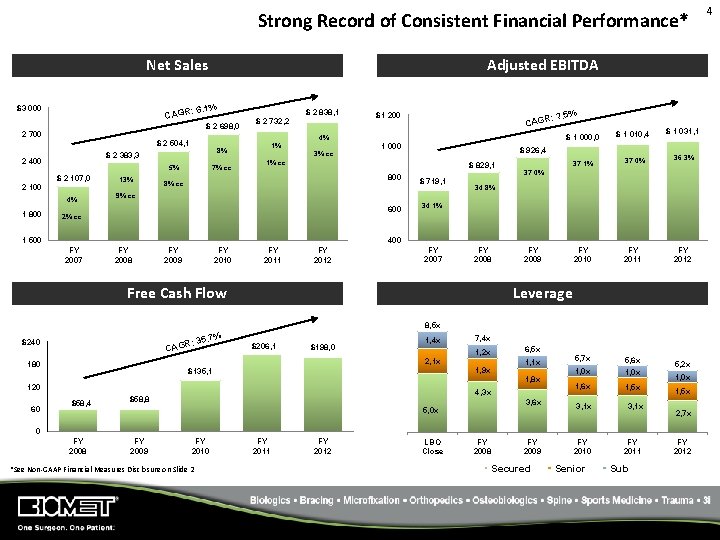
Strong Record of Consistent Financial Performance* Net Sales Adjusted EBITDA : 6. 1% CAGR $3 000 $ 2 698, 0 2 700 4% 1 800 7% cc 5% $ 2 107, 0 13% 1% 8% $ 2 383, 3 2 100 : 7. 5% $1 200 CAGR $ 1 000, 0 4% $ 2 504, 1 2 400 $ 2 838, 1 $ 2 732, 2 3% cc 1 000 $ 926, 4 1% cc $ 829, 1 800 8% cc $ 1 010, 4 $ 719, 1 $ 1 031, 1 37. 0% 36. 3% FY 2010 FY 2011 FY 2012 5, 7 x 5, 6 x 1, 0 x 1, 6 x 1, 5 x 37. 1% 37. 0% 34. 8% 9% cc 600 2% cc 34. 1% 400 1 500 FY 2007 FY 2008 FY 2009 FY 2010 FY 2011 FY 2012 FY 2007 FY 2008 Free Cash Flow FY 2009 Leverage 8, 5 x . 7% R: 35 $240 CAG $206, 1 $198, 0 1, 4 x 2, 1 x 180 120 60 1, 2 x 1, 9 x $135, 1 $58, 4 7, 4 x 6, 5 x 1, 1 x 1, 8 x 4, 3 x $58, 8 3, 6 x 5, 0 x 3, 1 x FY 2010 FY 2011 5, 2 x 1, 0 x 1, 5 x 2, 7 x 0 FY 2008 FY 2009 FY 2010 *See Non‐GAAP Financial Measures Disclosure on Slide 2 FY 2011 FY 2012 LBO Close FY 2008 FY 2009 Secured Senior Sub FY 2012 4

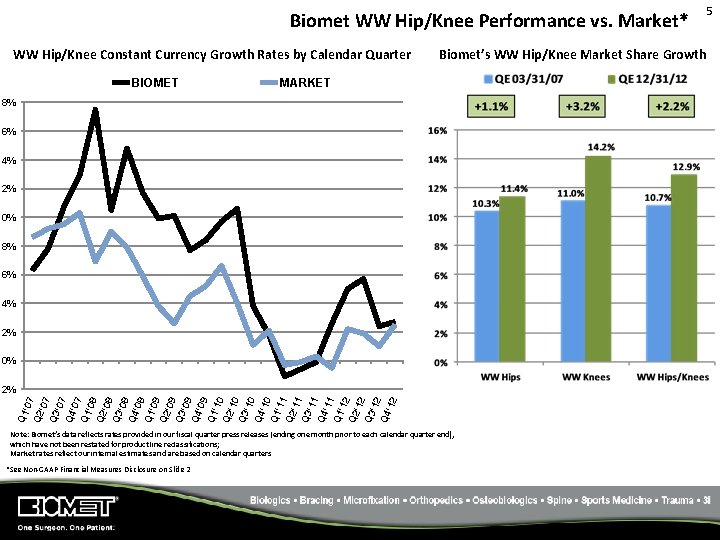
Biomet WW Hip/Knee Performance vs. Market* WW Hip/Knee Constant Currency Growth Rates by Calendar Quarter BIOMET Biomet’s WW Hip/Knee Market Share Growth MARKET 18% 16% 14% 12% 10% 8% 6% 4% 2% 0% Q 1 '07 Q 2 '07 Q 3 '07 Q 4 '07 Q 1 '08 Q 2 '08 Q 3 '08 Q 4 '08 Q 1 '09 Q 2 '09 Q 3 '09 Q 4 '09 Q 1 '10 Q 2 '10 Q 3 '10 Q 4 '10 Q 1 '11 Q 2 '11 Q 3 '11 Q 4 '11 Q 1 '12 Q 2 '12 Q 3 '12 Q 4 '12 -2% Note: Biomet’s data reflects rates provided in our fiscal quarter press releases (ending one month prior to each calendar quarter end), which have not been restated for product line reclassifications; Market rates reflect our internal estimates and are based on calendar quarters *See Non‐GAAP Financial Measures Disclosure on Slide 2 5

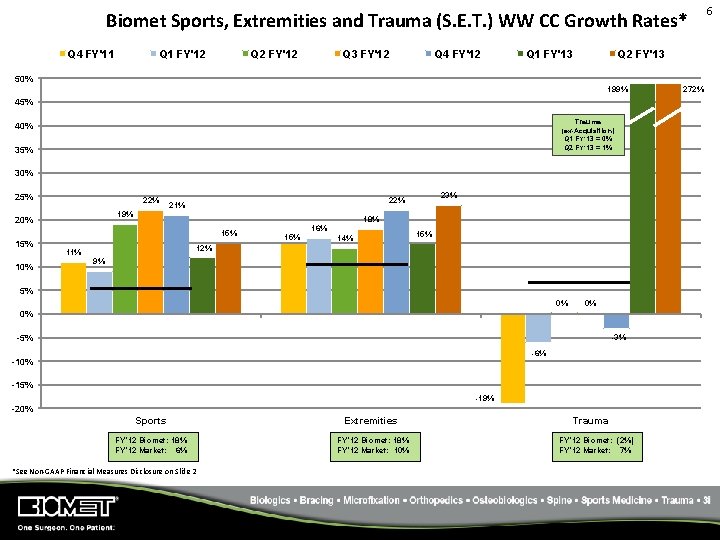
Biomet Sports, Extremities and Trauma (S. E. T. ) WW CC Growth Rates* Q 4 FY'11 Q 1 FY'12 Q 2 FY'12 Q 3 FY'12 Q 4 FY'12 Q 1 FY'13 Q 2 FY'13 50% 199% 45% Trauma (ex-Acquisition) Q 1 FY’ 13 = 0% Q 2 FY’ 13 = 1% 40% 35% 30% 25% 22% 19% 20% 18% 15% 10% 23% 22% 21% 15% 16% 14% 15% 12% 11% 9% 5% 0% 0% 0% -5% -3% -6% -10% -15% -19% -20% Sports Extremities FY’ 12 Biomet: 18% FY’ 12 Market: 6% FY’ 12 Biomet: 18% FY’ 12 Market: 10% *See Non‐GAAP Financial Measures Disclosure on Slide 2 Trauma FY’ 12 Biomet: (2%) FY’ 12 Market: 7% 272% 6

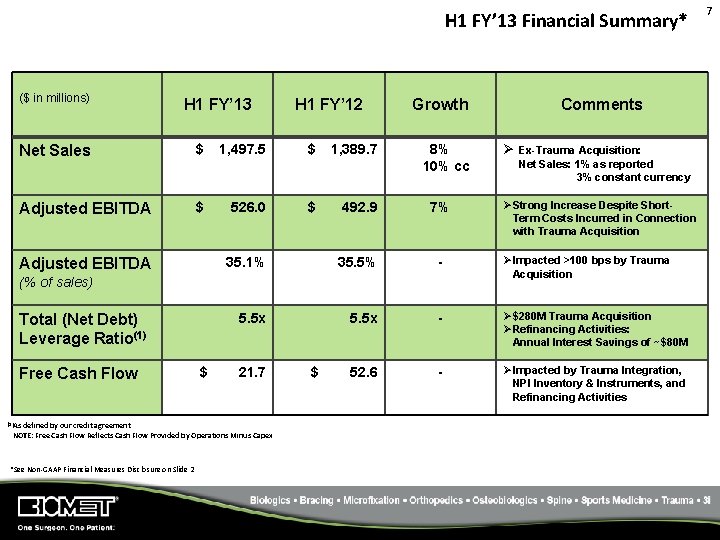
H 1 FY’ 13 Financial Summary* ($ in millions) H 1 FY’ 13 Net Sales $ Adjusted EBITDA 1, 497. 5 526. 0 H 1 FY’ 12 $ $ 1, 389. 7 Growth Comments 8% 10% cc Ø Ex-Trauma Acquisition: Free Cash Flow (1)As $ 7% 35. 1% 35. 5% - ØImpacted >100 bps by Trauma Acquisition 5. 5 x - Ø$280 M Trauma Acquisition ØRefinancing Activities: Annual Interest Savings of ~$80 M 52. 6 - ØImpacted by Trauma Integration, NPI Inventory & Instruments, and Refinancing Activities 21. 7 defined by our credit agreement NOTE: Free Cash Flow Reflects Cash Flow Provided by Operations Minus Capex *See Non‐GAAP Financial Measures Disclosure on Slide 2 ØStrong Increase Despite Short. Term Costs Incurred in Connection with Trauma Acquisition 492. 9 (% of sales) Total (Net Debt) Leverage Ratio(1) Net Sales: 1% as reported 3% constant currency $ 7

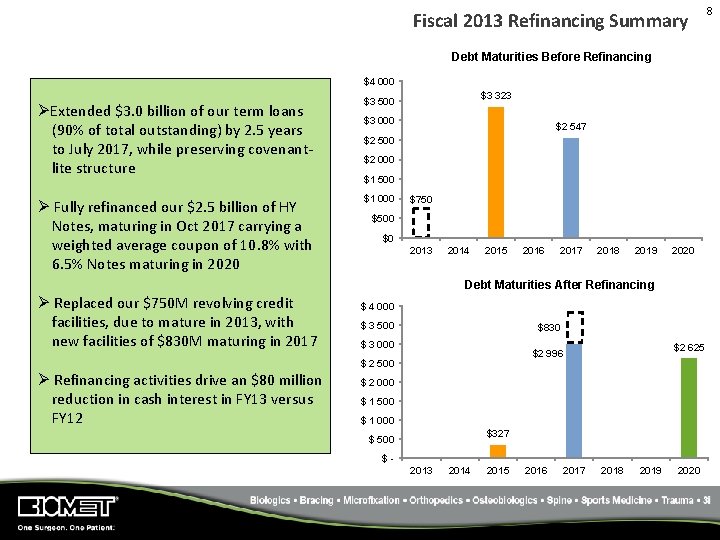
Fiscal 2013 Refinancing Summary Debt Maturities Before Refinancing $4 000 ØExtended $3. 0 billion of our term loans (90% of total outstanding) by 2. 5 years to July 2017, while preserving covenant‐ lite structure Ø Fully refinanced our $2. 5 billion of HY Notes, maturing in Oct 2017 carrying a weighted average coupon of 10. 8% with 6. 5% Notes maturing in 2020 $3 323 $3 500 $3 000 $2 547 $2 500 $2 000 $1 500 $1 000 $750 $500 $0 2013 2014 2015 2016 2017 2018 2019 2020 Debt Maturities After Refinancing Ø Replaced our $750 M revolving credit facilities, due to mature in 2013, with new facilities of $830 M maturing in 2017 $ 4 000 $ 3 500 $830 $ 3 000 $ 2 500 Ø Refinancing activities drive an $80 million reduction in cash interest in FY 13 versus FY 12 $2 625 $2 996 $ 2 000 $ 1 500 $ 1 000 $327 $ 500 $2013 2014 2015 2016 2017 2018 2019 2020 8

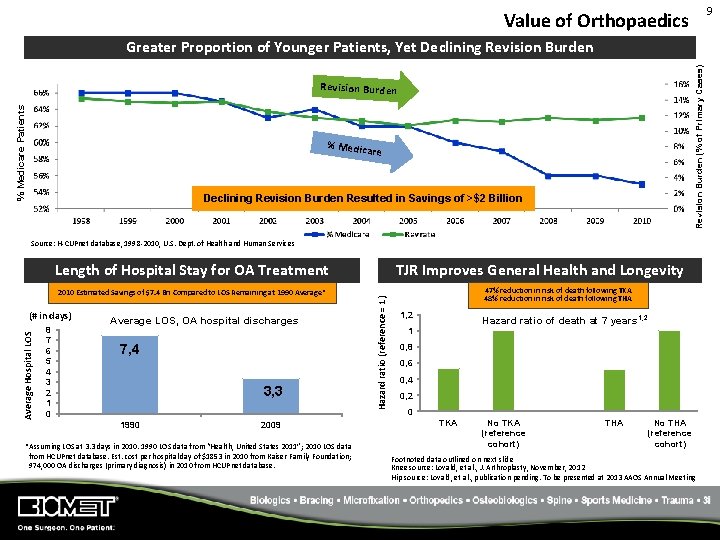
9 Value of Orthopaedics Revision Burden (% of Primary Cases) Greater Proportion of Younger Patients, Yet Declining Revision Burden % Medicare Patients Revision Burden % Medic are Declining Revision Burden Resulted in Savings of >$2 Billion Source: H‐CUPnet database, 1998‐ 2010, U. S. Dept. of Health and Human Services 2010 Estimated Savings of $7. 4 Bn Compared to LOS Remaining at 1990 Average* Average Hospital LOS (# in days) 8 7 6 5 4 3 2 1 0 Average LOS, OA hospital discharges 7, 4 3, 3 1990 2009 *Assuming LOS at 3. 3 days in 2010. 1990 LOS data from “Health, United States 2011”; 2010 LOS data from HCUPnet database. Est. cost per hospital day of $1853 in 2010 from Kaiser Family Foundation; 974, 000 OA discharges (primary diagnosis) in 2010 from HCUPnet database. TJR Improves General Health and Longevity Hazard ratio (reference = 1) Length of Hospital Stay for OA Treatment 47% reduction in risk of death following TKA 48% reduction in risk of death following THA 1, 2 Hazard ratio of death at 7 years 1, 2 1 0, 8 0, 6 0, 4 0, 2 0 TKA No TKA (reference cohort) THA No THA (reference cohort) Footnoted data outlined on next slide Knee source: Lovald, et al. , J. Arthroplasty, November, 2012 Hip source: Lovald, et al. , publication pending. To be presented at 2013 AAOS Annual Meeting

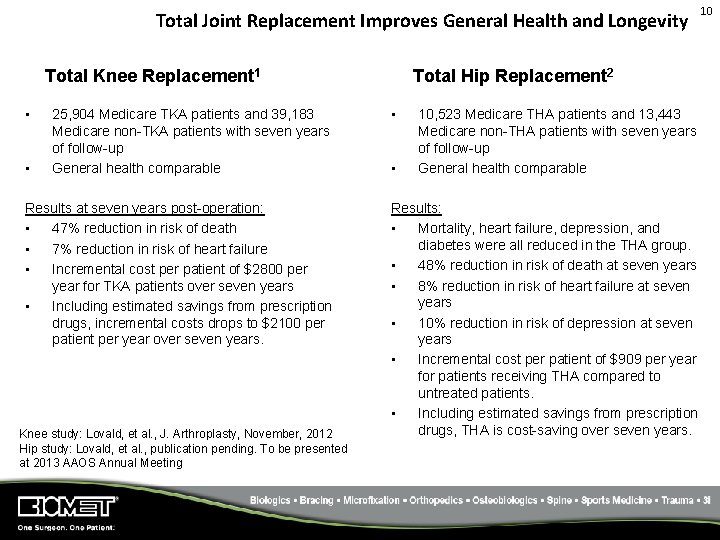
Total Joint Replacement Improves General Health and Longevity Total Knee Replacement 1 • • 25, 904 Medicare TKA patients and 39, 183 Medicare non-TKA patients with seven years of follow-up General health comparable Results at seven years post-operation: • 47% reduction in risk of death • 7% reduction in risk of heart failure • Incremental cost per patient of $2800 per year for TKA patients over seven years • Including estimated savings from prescription drugs, incremental costs drops to $2100 per patient per year over seven years. Knee study: Lovald, et al. , J. Arthroplasty, November, 2012 Hip study: Lovald, et al. , publication pending. To be presented at 2013 AAOS Annual Meeting Total Hip Replacement 2 • • 10, 523 Medicare THA patients and 13, 443 Medicare non-THA patients with seven years of follow-up General health comparable Results: • Mortality, heart failure, depression, and diabetes were all reduced in the THA group. • 48% reduction in risk of death at seven years • 8% reduction in risk of heart failure at seven years • 10% reduction in risk of depression at seven years • Incremental cost per patient of $909 per year for patients receiving THA compared to untreated patients. • Including estimated savings from prescription drugs, THA is cost-saving over seven years. 10

Keys to Winning in the Current Health Care Reform Environment ® Product Differentiation to Drive Share and Mix Increased Need for Clinical and Economic “Proof” Sources Service Offerings to Create Value Beyond Product Technology Focus on Pricing to Full Value 11

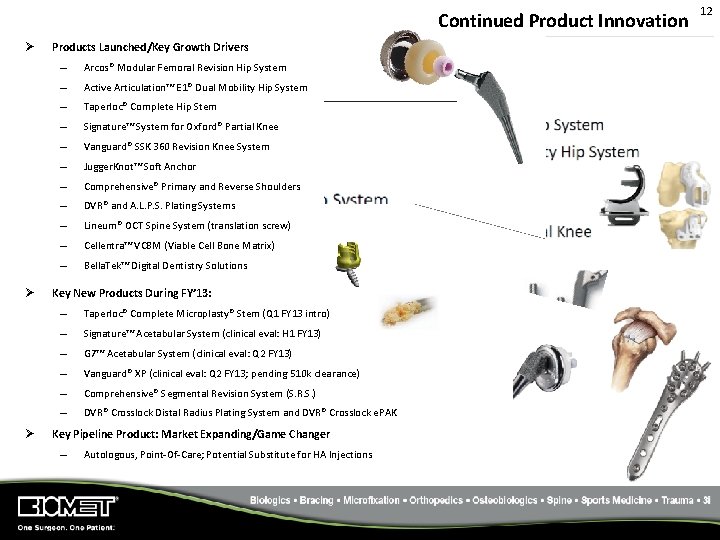
Continued Product Innovation Ø Ø Ø Products Launched/Key Growth Drivers – Arcos® Modular Femoral Revision Hip System – Active Articulation™ E 1® Dual Mobility Hip System – Taperloc® Complete Hip Stem – Signature™ System for Oxford® Partial Knee – Vanguard® SSK 360 Revision Knee System – Jugger. Knot™ Soft Anchor – Comprehensive® Primary and Reverse Shoulders – DVR® and A. L. P. S. Plating Systems – Lineum® OCT Spine System (translation screw) – Cellentra™ VCBM (Viable Cell Bone Matrix) – Bella. Tek™ Digital Dentistry Solutions Key New Products During FY’ 13: – Taperloc® Complete Microplasty® Stem (Q 1 FY 13 intro) – Signature™ Acetabular System (clinical eval: H 1 FY 13) – G 7™ Acetabular System (clinical eval: Q 2 FY 13) – Vanguard® XP (clinical eval: Q 2 FY 13; pending 510 k clearance) – Comprehensive® Segmental Revision System (S. R. S. ) – DVR® Crosslock Distal Radius Plating System and DVR® Crosslock e. PAK Key Pipeline Product: Market Expanding/Game Changer – Autologous, Point‐Of‐Care; Potential Substitute for HA Injections 12

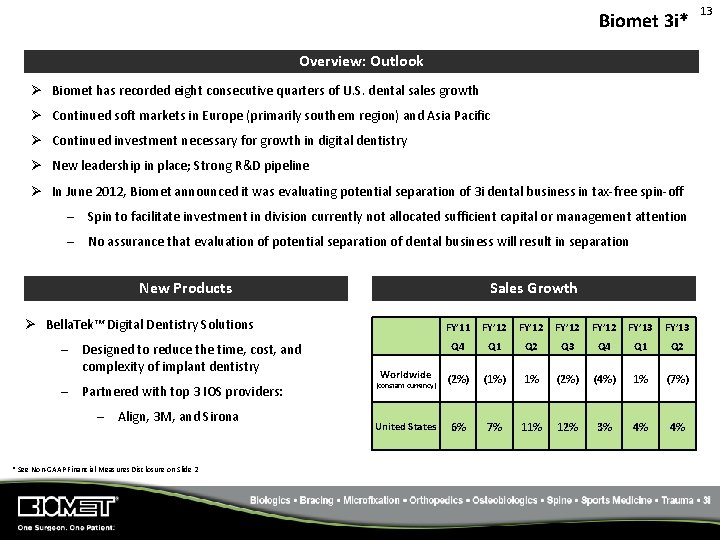
Biomet 3 i* Overview: Outlook Ø Biomet has recorded eight consecutive quarters of U. S. dental sales growth Ø Continued soft markets in Europe (primarily southern region) and Asia Pacific Ø Continued investment necessary for growth in digital dentistry Ø New leadership in place; Strong R&D pipeline Ø In June 2012, Biomet announced it was evaluating potential separation of 3 i dental business in tax‐free spin‐off – Spin to facilitate investment in division currently not allocated sufficient capital or management attention – No assurance that evaluation of potential separation of dental business will result in separation New Products Sales Growth Ø Bella. Tek™ Digital Dentistry Solutions – Designed to reduce the time, cost, and complexity of implant dentistry – Partnered with top 3 IOS providers: – Align, 3 M, and Sirona * See Non‐GAAP Financial Measures Disclosure on Slide 2 FY’ 11 FY’ 12 FY’ 13 Q 4 Q 1 Q 2 Q 3 Q 4 Q 1 Q 2 Worldwide (2%) (1%) 1% (2%) (4%) 1% (7%) United States 6% 7% 11% 12% 3% 4% 4% (constant currency) 13

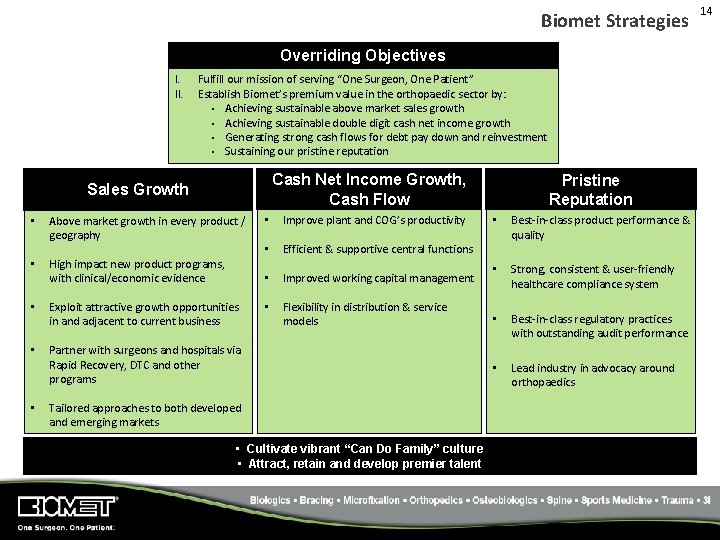
Biomet Strategies Overriding Objectives I. II. Fulfill our mission of serving “One Surgeon, One Patient” Establish Biomet’s premium value in the orthopaedic sector by: • Achieving sustainable above market sales growth • Achieving sustainable double digit cash net income growth • Generating strong cash flows for debt pay down and reinvestment • Sustaining our pristine reputation Cash Net Income Growth, Cash Flow Sales Growth • Above market growth in every product / geography • High impact new product programs, with clinical/economic evidence • Exploit attractive growth opportunities in and adjacent to current business • Partner with surgeons and hospitals via Rapid Recovery, DTC and other programs • • Improve plant and COG’s productivity • Efficient & supportive central functions • Improved working capital management • Flexibility in distribution & service models Tailored approaches to both developed and emerging markets • Cultivate vibrant “Can Do Family” culture • Attract, retain and develop premier talent Pristine Reputation • Best‐in‐class product performance & quality • Strong, consistent & user‐friendly healthcare compliance system • Best‐in‐class regulatory practices with outstanding audit performance • Lead industry in advocacy around orthopaedics 14

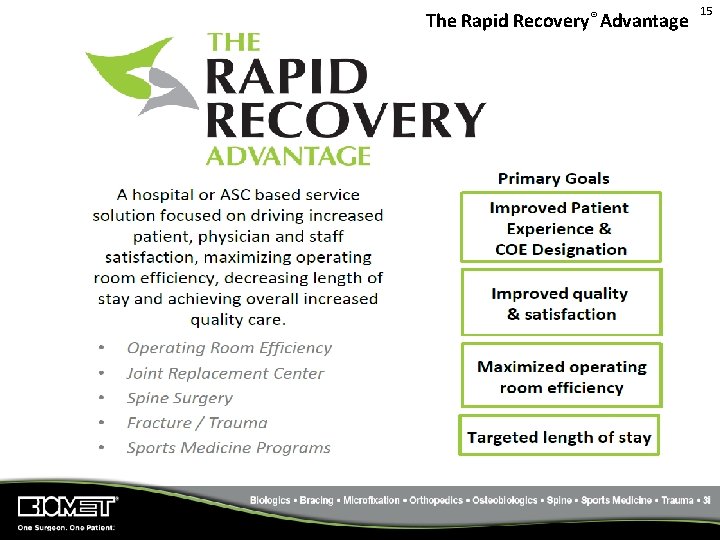
The Rapid Recovery® Advantage 15

Leader in Surgeon Education Ø Ø Ø Multiple Labs/Training Centers Ø Orthopaedic Skills Academy Ø Lorenz Skills Academy Ø Miami Lab Ø Parsippany Lab Ø Irvine Lab Courses Ø National Ø Regionally Focused Events Ø Cadaveric Ø Didactic Graduate Medical Education – GME Mobile Cadaveric Labs Surgeon Mentor Program Surgeon‐to‐Surgeon Visitation Program Tours Ø Surgeons Ø Residents and Fellows Ø C‐Suite Tradeshow Exhibitions Workshops at Third Party Events Biomet Mobile Learning Center (Sports Med Bus) 16

Value Creation Initiatives Value Creation Program Initiated Post LBO in Mid-2007 Has Delivered Significant Cash Savings (+$100 M) and EBITDA Contribution (+$65 M), While Maintaining the Highest Levels of Quality and Customer Service Ø Plant Network Optimization: Reduced Plant Facilities from 17 to 11 (including 2 new China plants) Ø Ø to Lower Cost of Production and Optimize Capacity Utilization Lean Manufacturing Strategic Sourcing SIOP and Inventory Management Program Now Embedded in Day to Day Operations Medical Device Tax (2. 3% of U. S. Sales) Adds Another Headwind…… Pursuing Additional Opportunities to Lower COGS & Improve our Supply Chain Ø Ø Emphasis on Plant‐Level KPIs Continue to Evaluate Plant Network in Light of Evolving Health Care Environment Lower Global Distribution/Warehousing Costs and E&O One Patient Solutions Initiative Improving Both Efficiency and Effectiveness in SG&A and R&D Ø Ø Global Orthopaedics Product Engine Structure Shared Service and Out‐Sourcing Models Ensure Sales Force Structure and Service Levels Provide Appropriate ROI Corp Infrastructure 17

Goldman Sachs Leveraged Finance Healthcare Conference 2013 March 6, 2013 Biomet, Inc. Daniel P. Florin Senior Vice President & Chief Financial Officer
 Stacy eastland goldman sachs
Stacy eastland goldman sachs Goldman sachs darkpool
Goldman sachs darkpool Goldman sachs puzzles
Goldman sachs puzzles Credit risk management and advisory goldman sachs
Credit risk management and advisory goldman sachs Goldman sachs
Goldman sachs Goldman sachs risk management framework
Goldman sachs risk management framework Goldman sachs 10 000 small businesses requirements
Goldman sachs 10 000 small businesses requirements Sean brennan goldman sachs
Sean brennan goldman sachs Goldman sachs commodities research
Goldman sachs commodities research Goldman sachs partner compensation plan
Goldman sachs partner compensation plan Goldman sachs mask
Goldman sachs mask Goldman sachs organizational structure
Goldman sachs organizational structure Goldman sachs case competition
Goldman sachs case competition Goldman sachs
Goldman sachs Goldman sachs veterans integration program
Goldman sachs veterans integration program Money multiplier
Money multiplier Icon sachs
Icon sachs Poland national anthem lyrics
Poland national anthem lyrics Lpc collateral
Lpc collateral