Geochemical Cycles Water Cycle Movement of water among












- Slides: 18

Geochemical Cycles

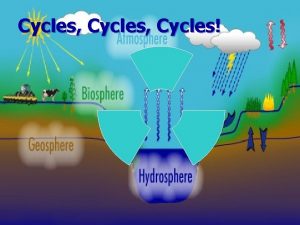
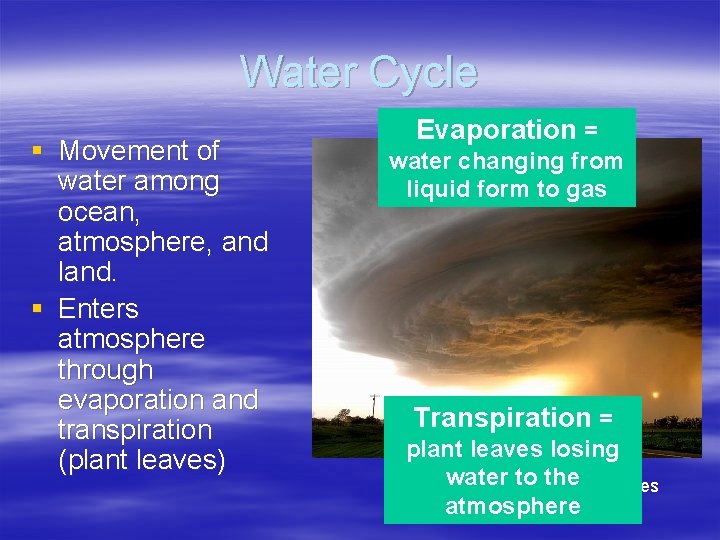
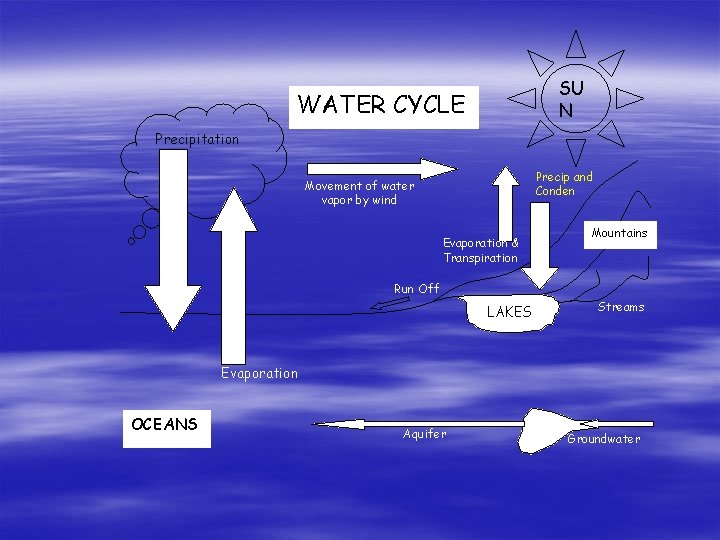
Water Cycle § Movement of water among ocean, atmosphere, and land. § Enters atmosphere through evaporation and transpiration (plant leaves) Evaporation = water changing from liquid form to gas Transpiration = plant leaves losing water. Katrina to the Hurricane approaches atmosphere

Geochemical Cycles § § § Water Cycle When air is warmed up, the particles get farther apart (and so have lower density). H 20 rises in columns of warm air and may remain in atmosphere for about 2 weeks. As the H 20 vapor rises, it cools into droplets (condenses), forming clouds Condensation = water vapor transforming into liquid water. Occurs because cooler air does not have as much space to hold water vapor.

Water Cycle § Enters land through precipitation and condensation. § Enters lakes or rivers through runoff § Enters groundwater where it enters the biosphere. Runoff = any water moving across the land Groundwater = any water stored underground! When water vapor in the air cools (usually at night), it condenses on grass (dew) or in the air (fog).

SU N WATER CYCLE Precipitation Precip and Conden Movement of water vapor by wind Evaporation & Transpiration Mountains Run Off LAKES Streams Evaporation OCEANS Aquifer Groundwater

Humans affect the water cycle § Higher global temperature increased evaporation. § Higher ocean temps increase evaporation § Reduction in rainforest reduces transpiration. § Reduction of plant life increases runoff § Glacial melting reduces amount of reflected light

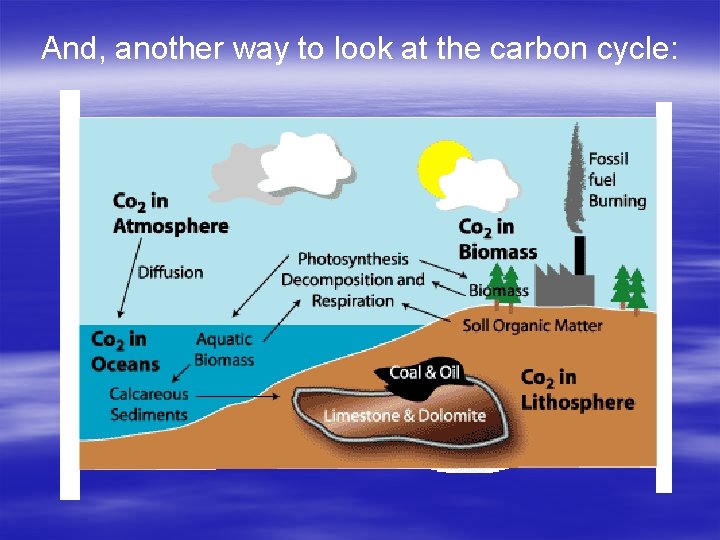
Geochemical Cycles Carbon Cycle § Early atmosphere of Earth 95% CO 2. Photosynthetic plants removed some of the CO 2 and added O 2. Today’s atmosphere is 0. 04% CO 2! Carbon is found in the § Reactions of photosynthesis and cellular atmosphere primarily as CO 2 respiration couldn’t take place without carbon. These two reactions form a continuous cycle. § Two important sources of Carbon are the ocean (since CO 2 dissolves easily in H 20)Respiration and rocks : Photosynthesis : Cellular (such as coal, oreofand. Organisms limestonetake formed fromand in Plants taking CO 2 out that sugar dead organisms) the atmosphere and the process of burning energy using it to produce sugar. release CO 2 back into the atmosphere.

And, another way to look at the carbon cycle:

Humans affect the Carbon Cycle § Burning of fossil fuels, (oil, coal and natural gas). § Fossil fuels were formed very long ago and is “fixed”: essentially locked out of the carbon cycle. § By burning fossil fuels the carbon is released back into the cycle.

Humans affect the Carbon Cycle § We presently release more carbon into the air than can be reabsorbed by photosynthetic organisms, thereby we have a net INCREASE of carbon in the cycle. § This atmospheric carbon has a role to play in the warming of the atmosphere.

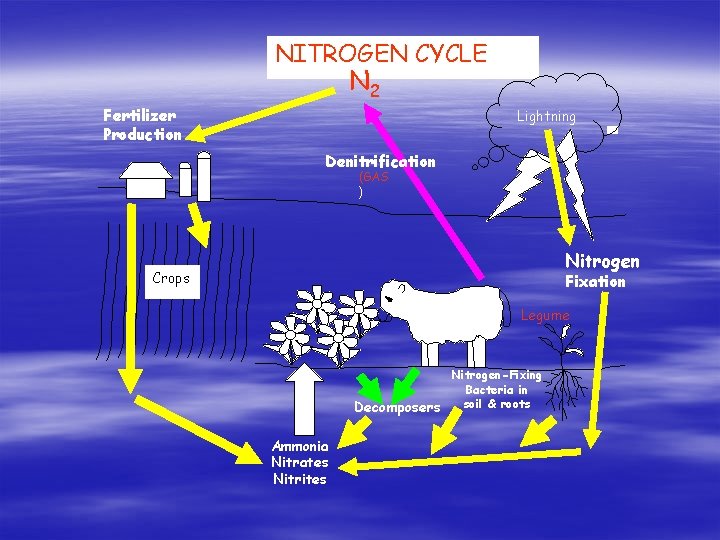
Geochemical Cycles § § Nitrogen Cycle Organisms require Nitrogen to form amino acids for the building of proteins. Lots of N 2 in our atmosphere Unfortunately, most organisms CANNOT use atmospheric nitrogen. Nitrogen-fixing bacteria CAN use N 2 from the atmosphere. Nitrogen-fixing bacteria convert atmospheric N 2 into ammonia (NH 4) which is a form of nitrogen that plants CAN use.

Nitrogen Cycle Continued … § Nitrogen-fixing bacteria live in the soil and in roots of legumes. § These bacteria also form nitrites (NO 2) and nitrates (NO 3); which are compounds containing N and O. § Nitrate is the most common source of N for plants. § Animals get N from the proteins they eat. § Decomposers return N to the soil in the form of ammonia and the cycle repeats. § So, oftentimes, the nitrogen cycle does not require the N to be returned to atmospheric form!

Nitrogen Cycle Summary § All living organisms require nitrogen – to form amino acids to build proteins. § Proteins are important for locomotion, reproduction, defense, and structure. § Nitrogen makes up 78% of atmosphere as N 2 § Nitrogen-fixing bacteria are very important N 2 needs to be “fixed” before it can be used by most living things.

NITROGEN CYCLE N 2 Fertilizer Production Lightning Denitrification (GAS ) Nitrogen Crops Fixation Sheep Legume Nitrogen-Fixing Bacteria in soil & roots Decomposers Ammonia Nitrates Nitrites


Humans affect the Nitrogen Cycle § From the production and use of nitrogen fertilizers to the burning of fossil fuels in automobiles, power plants, and industries, humans impact this cycle. § Nitrogen is essential to living organisms and its availability plays a crucial role in the world's ecosystems. § Excessive nitrogen additions can pollute ecosystems

Humans affect the Nitrogen Cycle § Increased global concentrations of nitrous oxide (N 2 O), a potent greenhouse gas, in the atmosphere § Increased concentrations of nitric oxide, (NO) that drive the formation of smog along with N 2 O § Losses of soil nutrients such as calcium and potassium that are essential for long-term soil fertility

Humans affect the Nitrogen Cycle § Acidification of soils and of the waters of streams and lakes § Greatly increased transport of nitrogen by rivers into estuaries and coastal waters where it is a major pollutant.
 Practice geochemical cycles answer key
Practice geochemical cycles answer key Primary and secondary geochemical dispersion
Primary and secondary geochemical dispersion Biogeochemical cycles water cycle
Biogeochemical cycles water cycle Water and water and water water
Water and water and water water Biogeochemical cycles of water
Biogeochemical cycles of water Water cycles of matter
Water cycles of matter Water cycle brainpop
Water cycle brainpop Water cycle the hydrologic cycle
Water cycle the hydrologic cycle Non-movement area
Non-movement area 5 axial steps
5 axial steps Mandarin cycles
Mandarin cycles My tippet – only tulle –
My tippet – only tulle – Pdsa cycle nursing
Pdsa cycle nursing Precession milankovitch cycles
Precession milankovitch cycles Similarities between frog and butterfly
Similarities between frog and butterfly Relationship between transaction cycles
Relationship between transaction cycles Cpi cycles per instruction
Cpi cycles per instruction Biogeochemical cycles apes
Biogeochemical cycles apes Abiotic cycles
Abiotic cycles