General Physics II Applications of Quantum Mechanics Apr


- Slides: 9

General Physics II Applications of Quantum Mechanics Apr 13, 2007 PHYS 142 1

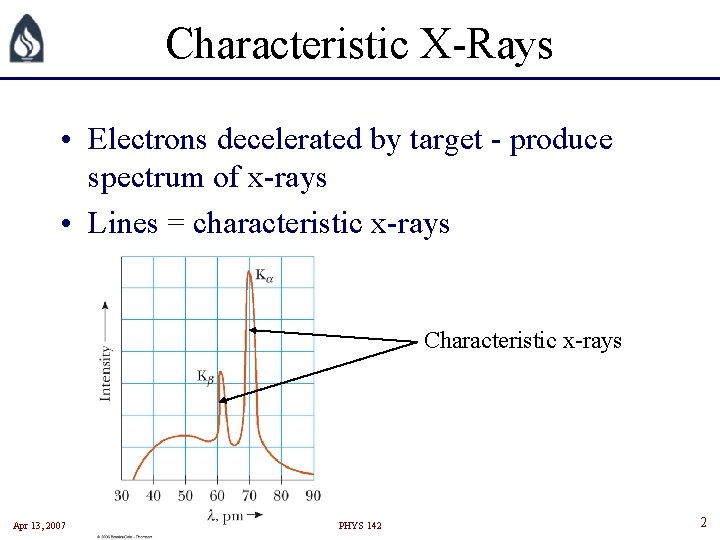
Characteristic X-Rays • Electrons decelerated by target - produce spectrum of x-rays • Lines = characteristic x-rays Characteristic x-rays Apr 13, 2007 PHYS 142 2

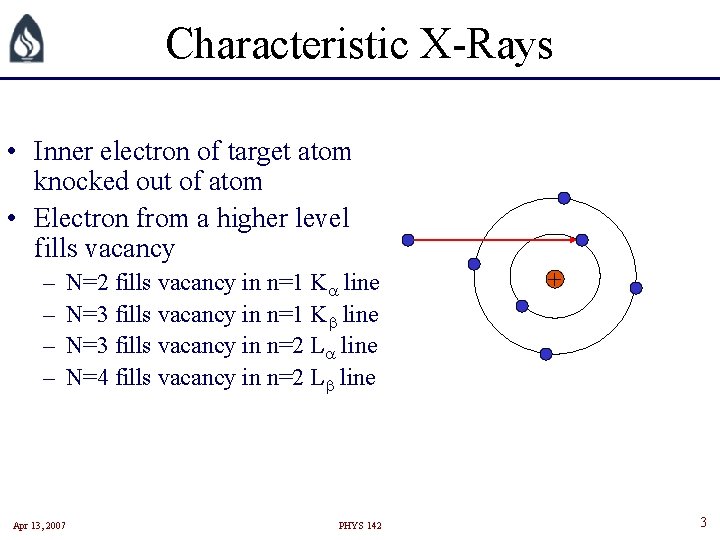
Characteristic X-Rays • Inner electron of target atom knocked out of atom • Electron from a higher level fills vacancy – – N=2 fills vacancy in n=1 K line N=3 fills vacancy in n=2 L line N=4 fills vacancy in n=2 L line Apr 13, 2007 PHYS 142 + 3

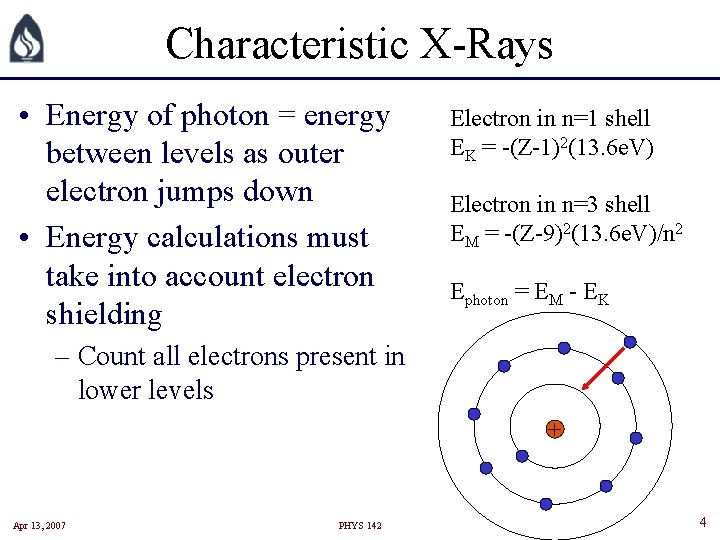
Characteristic X-Rays • Energy of photon = energy between levels as outer electron jumps down • Energy calculations must take into account electron shielding Electron in n=1 shell EK = -(Z-1)2(13. 6 e. V) Electron in n=3 shell EM = -(Z-9)2(13. 6 e. V)/n 2 Ephoton = EM - EK – Count all electrons present in lower levels + Apr 13, 2007 PHYS 142 4

Example 1 • An electron drops from the M shell (n=3) to a vacancy in the K shell (n=1) in a silver Z=47 atom. What is the change in energy Ei - E f? Apr 13, 2007 PHYS 142 5

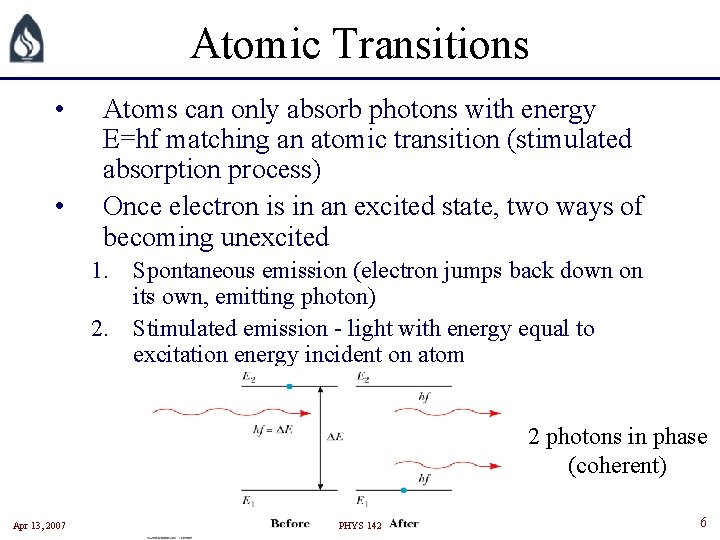
Atomic Transitions • • Atoms can only absorb photons with energy E=hf matching an atomic transition (stimulated absorption process) Once electron is in an excited state, two ways of becoming unexcited 1. Spontaneous emission (electron jumps back down on its own, emitting photon) 2. Stimulated emission - light with energy equal to excitation energy incident on atom 2 photons in phase (coherent) Apr 13, 2007 PHYS 142 6

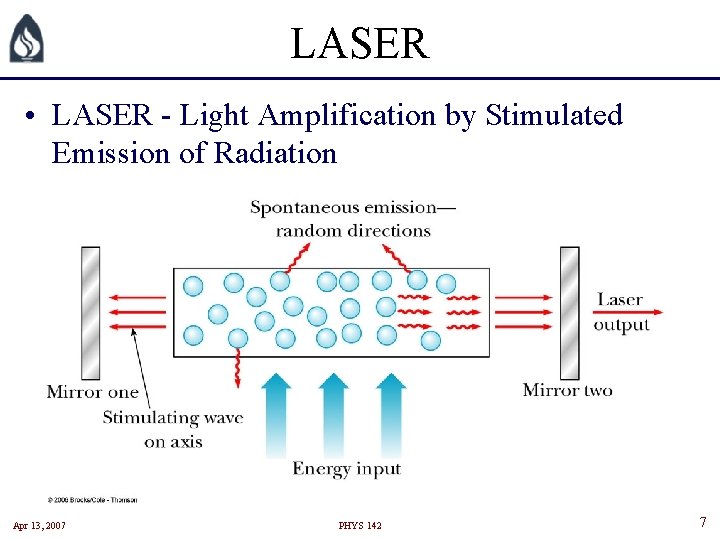
LASER • LASER - Light Amplification by Stimulated Emission of Radiation Apr 13, 2007 PHYS 142 7

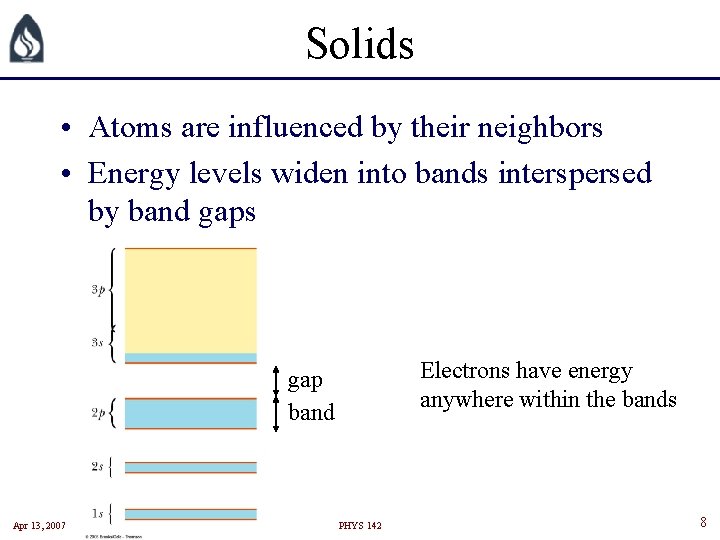
Solids • Atoms are influenced by their neighbors • Energy levels widen into bands interspersed by band gaps Electrons have energy anywhere within the bands gap band Apr 13, 2007 PHYS 142 8

Example 2 • When a hydrogen atom absorbs a photon that raises it to the n=4 state, what is the greatest number of photons that can be emitted as it drops back to the ground state? Apr 13, 2007 PHYS 142 9
 Quantum physics vs mechanics
Quantum physics vs mechanics Quantum physics vs quantum mechanics
Quantum physics vs quantum mechanics Beta positive decay
Beta positive decay Is sinx acceptable wave function
Is sinx acceptable wave function Expectation value of energy in quantum mechanics
Expectation value of energy in quantum mechanics Expectation value in quantum mechanics
Expectation value in quantum mechanics Expectation value of energy in quantum mechanics
Expectation value of energy in quantum mechanics French and taylor quantum mechanics
French and taylor quantum mechanics Quantum mechanics in your face
Quantum mechanics in your face Postulates of quantum mechanics
Postulates of quantum mechanics