GENE EDITING Regulatory IP Considerations Cassie J Edgar



- Slides: 10

GENE EDITING: Regulatory & IP Considerations Cassie J. Edgar Chief IP & Regulatory Officer Genus plc Iowa. BIO Innovation Workshop November 2017

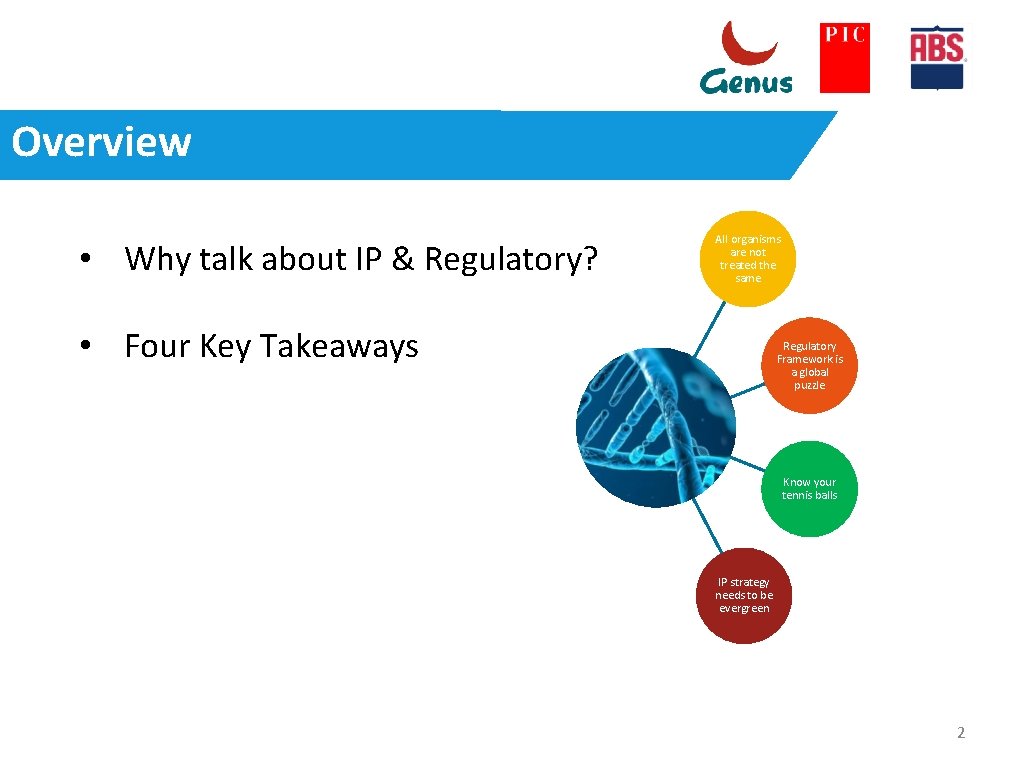
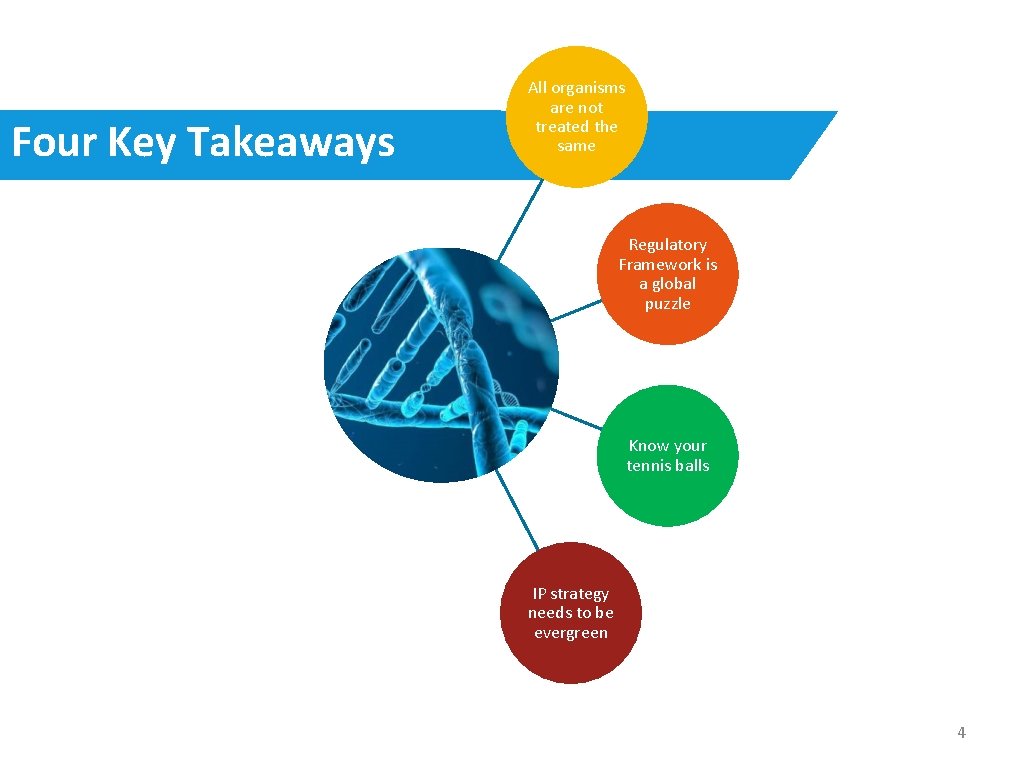
Overview • Why talk about IP & Regulatory? All organisms are not treated the same • Four Key Takeaways Regulatory Framework is a global puzzle Know your tennis balls IP strategy needs to be evergreen 2

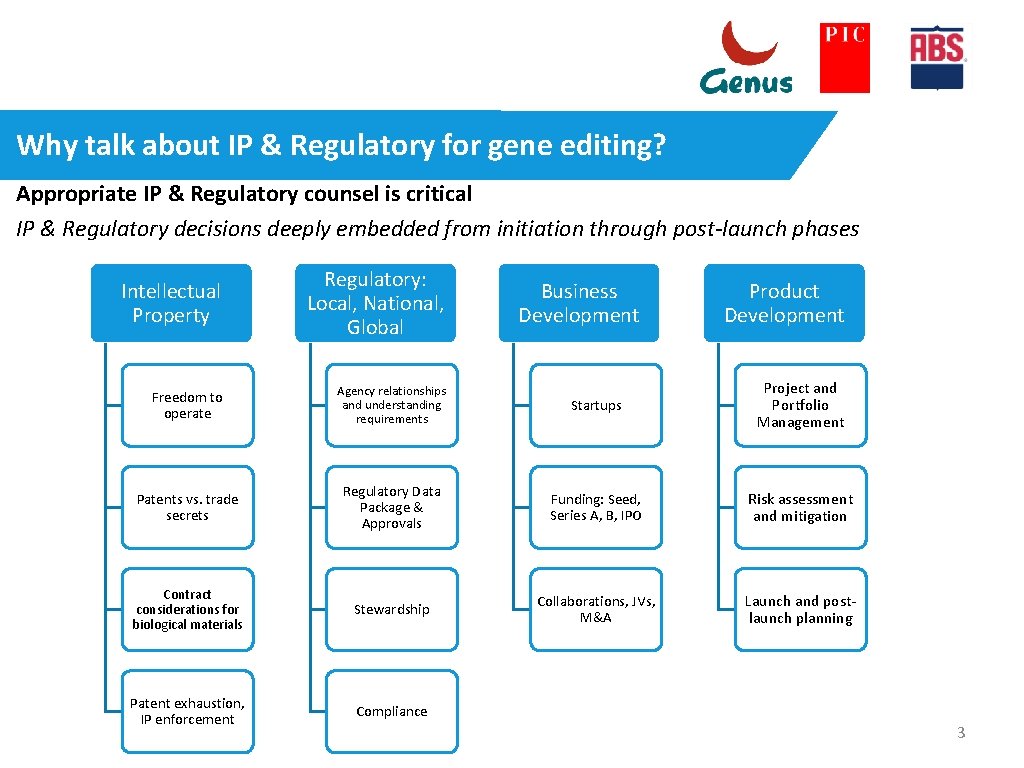
Why talk about IP & Regulatory for gene editing? Appropriate IP & Regulatory counsel is critical IP & Regulatory decisions deeply embedded from initiation through post-launch phases Intellectual Property Regulatory: Local, National, Global Freedom to operate Agency relationships and understanding requirements Startups Project and Portfolio Management Patents vs. trade secrets Regulatory Data Package & Approvals Funding: Seed, Series A, B, IPO Risk assessment and mitigation Contract considerations for biological materials Stewardship Collaborations, JVs, M&A Launch and postlaunch planning Patent exhaustion, IP enforcement Compliance Business Development Product Development 3

Four Key Takeaways All organisms are not treated the same Regulatory Framework is a global puzzle Know your tennis balls IP strategy needs to be evergreen 4

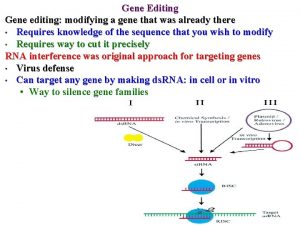
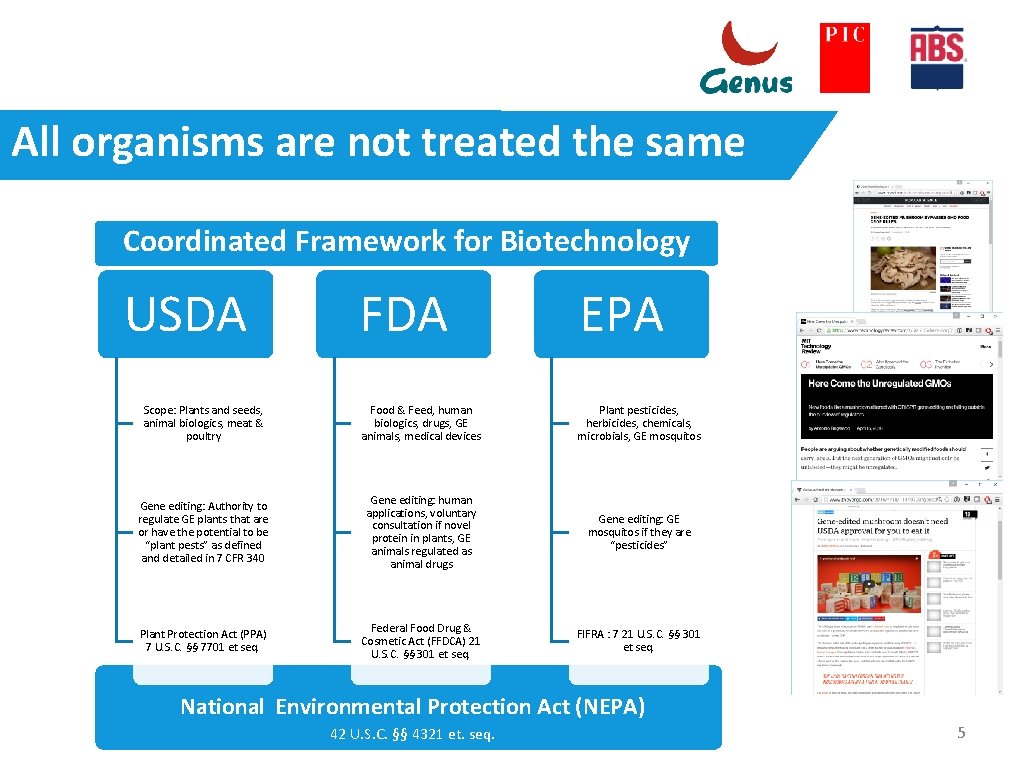
All organisms are not treated the same Coordinated Framework for Biotechnology USDA FDA EPA Scope: Plants and seeds, animal biologics, meat & poultry Food & Feed, human biologics, drugs, GE animals, medical devices Plant pesticides, herbicides, chemicals, microbials, GE mosquitos Gene editing: Authority to regulate GE plants that are or have the potential to be “plant pests” as defined and detailed in 7 CFR 340 Gene editing: human applications, voluntary consultation if novel protein in plants, GE animals regulated as animal drugs Gene editing: GE mosquitos if they are “pesticides” Plant Protection Act (PPA) 7 U. S. C. §§ 7701 et seq. Federal Food Drug & Cosmetic Act (FFDCA) 21 U. S. C. §§ 301 et seq. FIFRA : 7 21 U. S. C. §§ 301 et seq. National Environmental Protection Act (NEPA) 42 U. S. C. §§ 4321 et. seq. 5

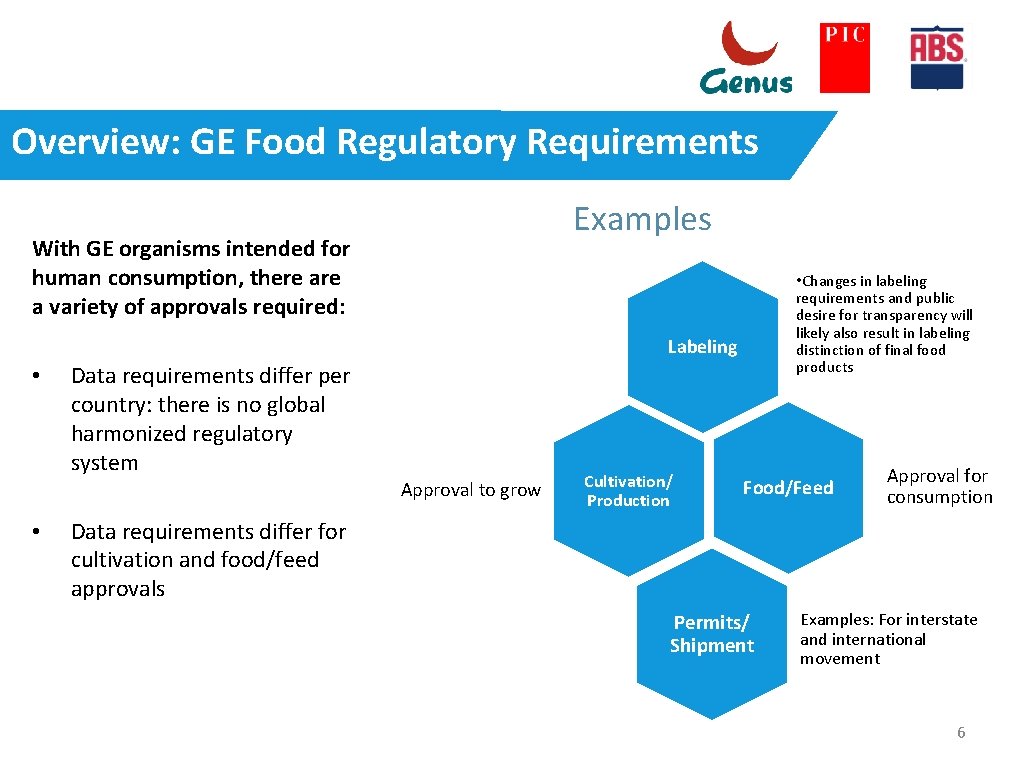
Overview: GE Food Regulatory Requirements Examples With GE organisms intended for human consumption, there a variety of approvals required: • Changes in labeling requirements and public desire for transparency will likely also result in labeling distinction of final food products Labeling • Data requirements differ per country: there is no global harmonized regulatory system Approval to grow • Cultivation/ Production Food/Feed Approval for consumption Data requirements differ for cultivation and food/feed approvals Permits/ Shipment Examples: For interstate and international movement 6

Regulatory framework for gene editing is global puzzle • Engagement with emerging global regulatory framework is necessary for commodity products • Countries are still figuring out when and how to regulate gene edited products • Engagement in dialogue is critical With public, government, industry groups, and regulators Recent jurisdictions of focus: Canada, Mexico, China, Korea, Japan, EU 7

Know your tennis balls IP analysis and strategy begins with answering this question: “What are you trying to accomplish” • Consider offense and defense • News on interference proceedings or patent claims or “who owns the patents” must be analyzed in the proper context of that question Ball holders Keeping score Tennis Balls Footballs Green tennis balls Methods of playing the game 8

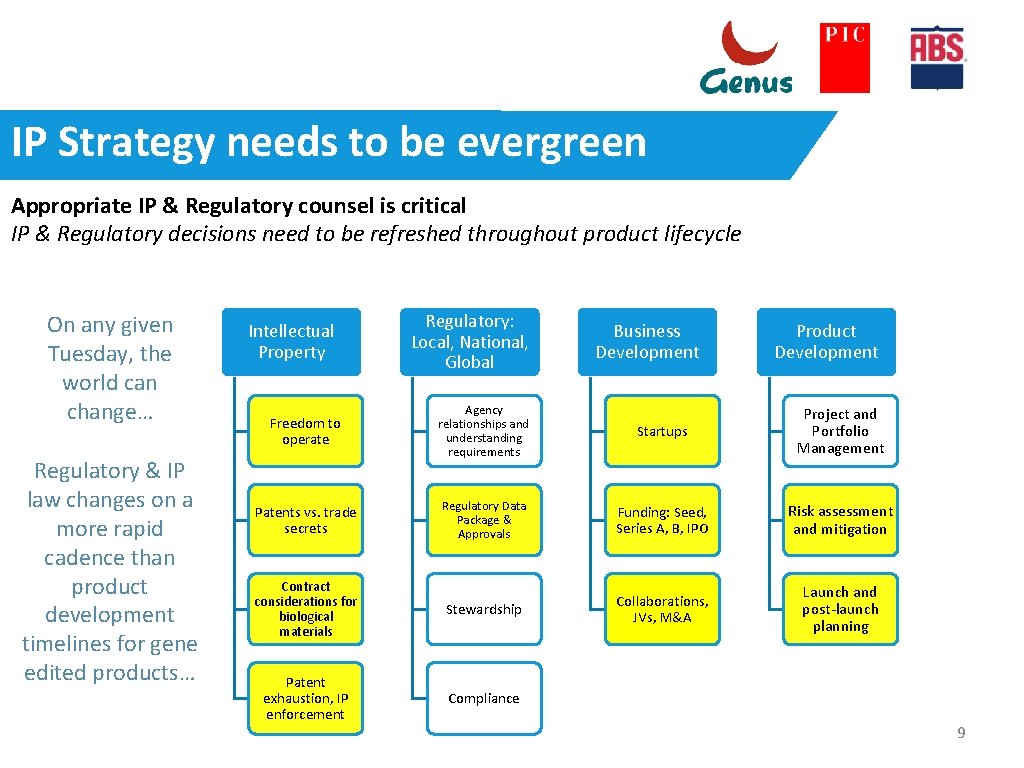
IP Strategy needs to be evergreen Appropriate IP & Regulatory counsel is critical IP & Regulatory decisions need to be refreshed throughout product lifecycle On any given Tuesday, the world can change… Regulatory & IP law changes on a more rapid cadence than product development timelines for gene edited products… Intellectual Property Regulatory: Local, National, Global Business Development Product Development Freedom to operate Agency relationships and understanding requirements Startups Project and Portfolio Management Patents vs. trade secrets Regulatory Data Package & Approvals Funding: Seed, Series A, B, IPO Risk assessment and mitigation Contract considerations for biological materials Stewardship Collaborations, JVs, M&A Launch and post-launch planning Patent exhaustion, IP enforcement Compliance 9

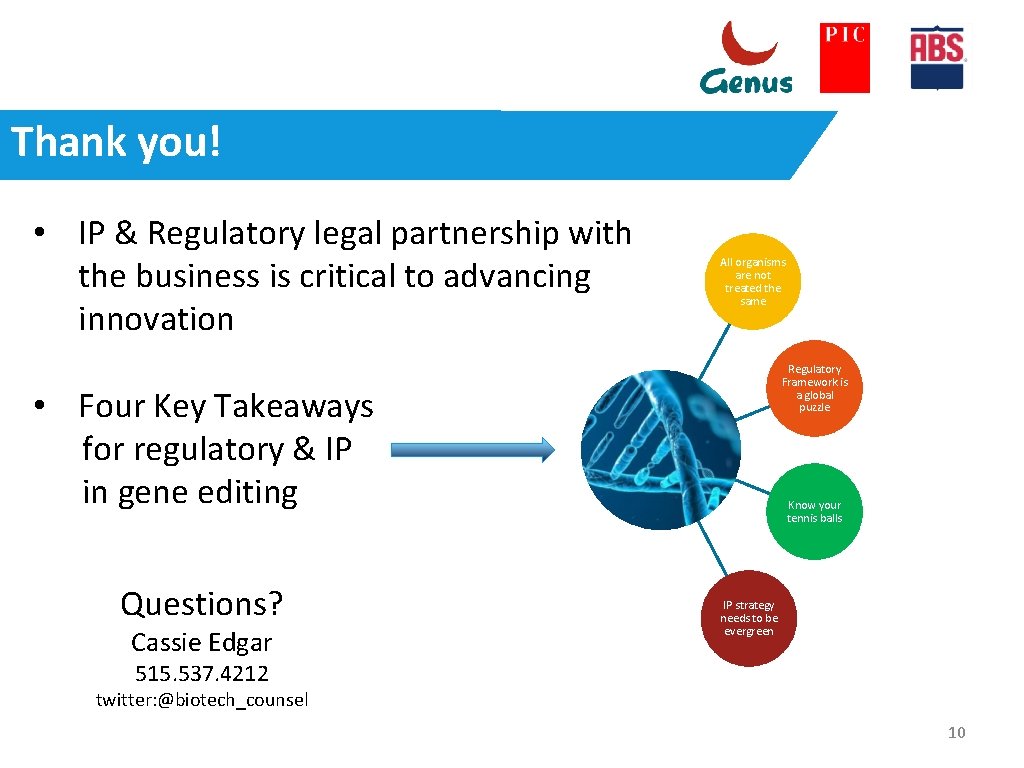
Thank you! • IP & Regulatory legal partnership with the business is critical to advancing innovation All organisms are not treated the same Regulatory Framework is a global puzzle • Four Key Takeaways for regulatory & IP in gene editing Questions? Cassie Edgar Know your tennis balls IP strategy needs to be evergreen 515. 537. 4212 twitter: @biotech_counsel 10