Final Physics 101 Lecture 26 Thermodynamics II Physics


- Slides: 10

Final Physics 101: Lecture 26 Thermodynamics II Physics 101: Lecture 26, Pg 1

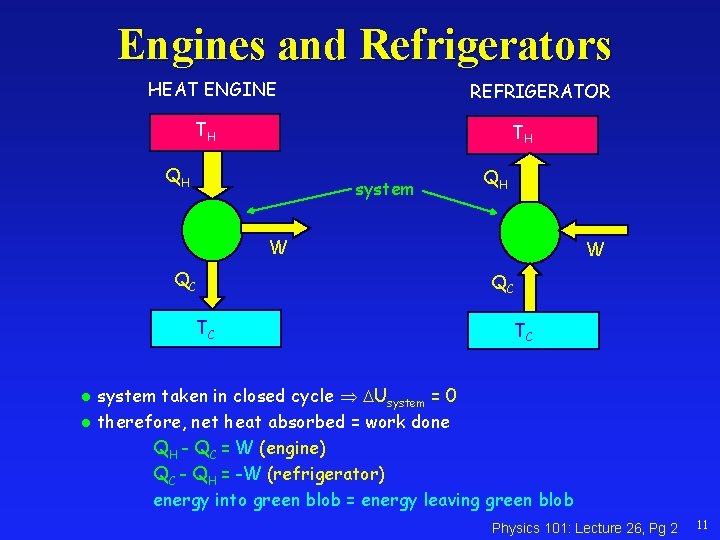
Engines and Refrigerators HEAT ENGINE REFRIGERATOR TH TH QH system QH W QC TC system taken in closed cycle Usystem = 0 l therefore, net heat absorbed = work done QH - QC = W (engine) QC - QH = -W (refrigerator) energy into green blob = energy leaving green blob l Physics 101: Lecture 26, Pg 2 11

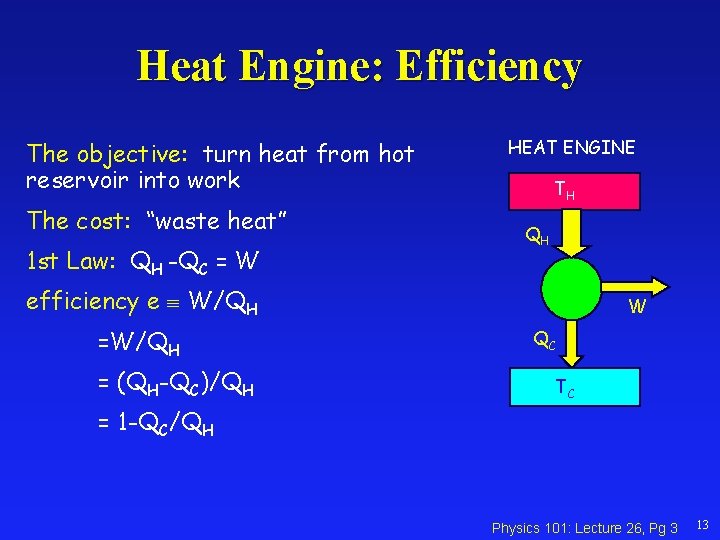
Heat Engine: Efficiency The objective: turn heat from hot reservoir into work The cost: “waste heat” 1 st Law: QH -QC = W HEAT ENGINE TH QH efficiency e W/QH = (QH-QC)/QH W QC TC = 1 -QC/QH Physics 101: Lecture 26, Pg 3 13

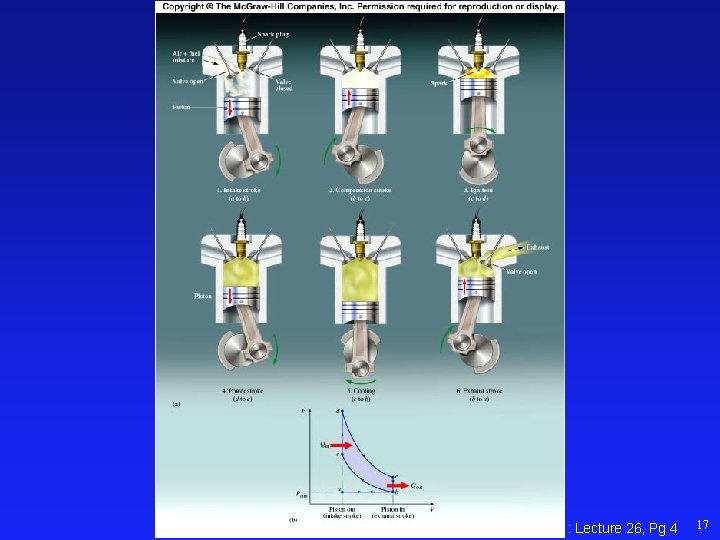
Physics 101: Lecture 26, Pg 4 17

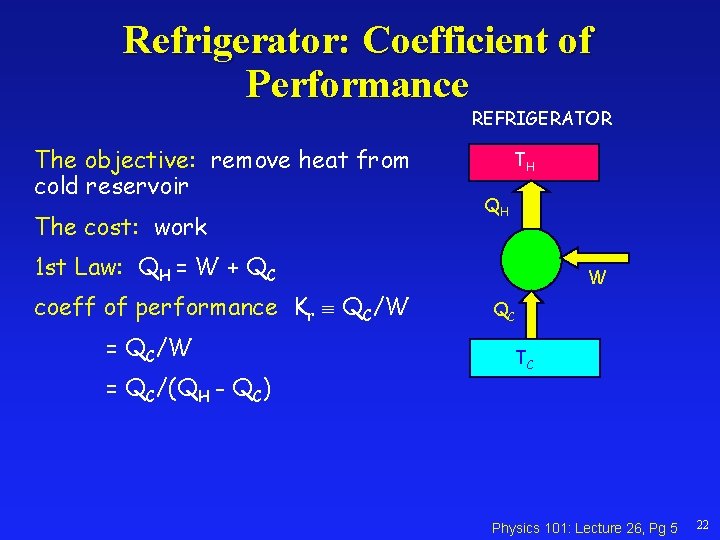
Refrigerator: Coefficient of Performance REFRIGERATOR The objective: remove heat from cold reservoir The cost: work TH QH 1 st Law: QH = W + QC coeff of performance Kr QC/W = QC/(QH - QC) W QC TC Physics 101: Lecture 26, Pg 5 22

New concept: Entropy (S) l A measure of “disorder” l A property of a system (just like p, V, T, U) èrelated to number of different “states” of system l Examples of increasing entropy: èice cube melts ègases expand into vacuum l Change in entropy: è S = Q/T » >0 if heat flows into system (Q>0) » <0 if heat flows out of system (Q<0) Physics 101: Lecture 26, Pg 6 25

Second Law of Thermodynamics l The entropy change (Q/T) of the system+environment 0 ènever < 0 èorder to disorder l Consequences èA “disordered” state cannot spontaneously transform into an “ordered” state èNo engine operating between two reservoirs can be more efficient than one that produces 0 change in entropy. This is called a “Carnot engine” Physics 101: Lecture 26, Pg 7 31

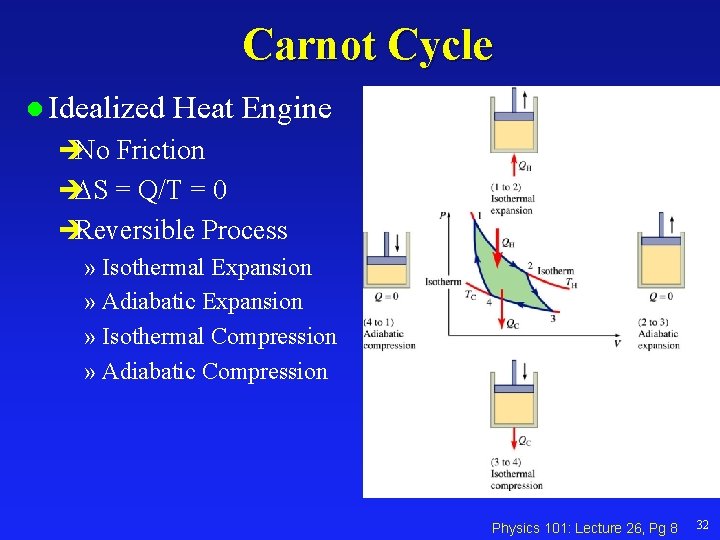
Carnot Cycle l Idealized Heat Engine èNo Friction è S = Q/T = 0 èReversible Process » Isothermal Expansion » Adiabatic Expansion » Isothermal Compression » Adiabatic Compression Physics 101: Lecture 26, Pg 8 32

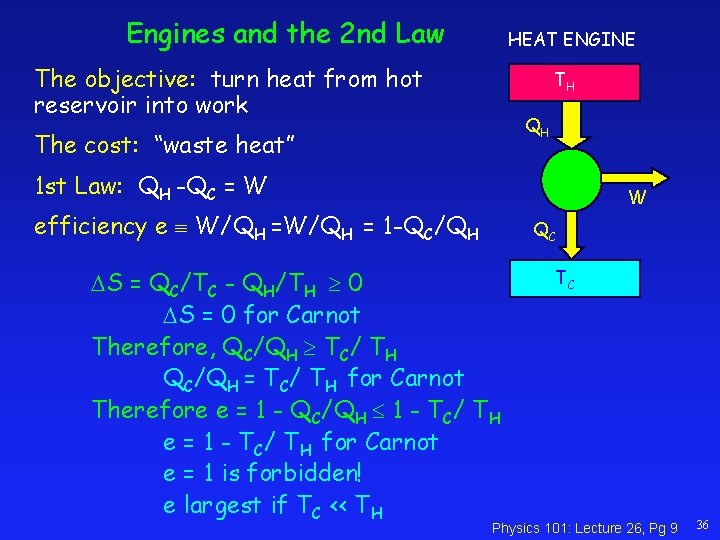
Engines and the 2 nd Law HEAT ENGINE The objective: turn heat from hot reservoir into work TH QH The cost: “waste heat” 1 st Law: QH -QC = W W efficiency e W/QH = 1 -QC/QH QC S = QC/TC - QH/TH 0 S = 0 for Carnot Therefore, QC/QH TC/ TH QC/QH = TC/ TH for Carnot Therefore e = 1 - QC/QH 1 - TC/ TH e = 1 - TC/ TH for Carnot e = 1 is forbidden! e largest if TC << TH TC Physics 101: Lecture 26, Pg 9 36

Summary l First Law of thermodynamics: Energy Conservation èQ = U + W l Heat Engines èEfficiency = = 1 -QC/QH l Refrigerators èCoefficient of Performance = QC/(QH - QC) S = Q/T l Entropy l 2 nd Law: Entropy always increases! Carnot Cycle: Reversible, Maximum Efficiency e = 1 – Tc/Th l Physics 101: Lecture 26, Pg 10 50