FDA Hearing on Drug Safety and the Rosiglitazone









- Slides: 14

FDA Hearing on Drug Safety and the Rosiglitazone Controversy Richard Hellman, MD, FACP, FACE President, American Association of Clinical Endocrinologists Clinical Professor of Medicine University of Missouri/Kansas City School of Medicine Member, Executive Committee Physician Consortium for Performance Improvement July 30, 2007

The Unanswered Questions Regarding Rosiglitazone n n Why were epidemiologic studies regarding special high risk populations not yet complete? Why were there relatively few comparative studies of efficacy? Why were the clinical trials available not adequately powered to test for cardiovascular risk? Were these shortcomings in safety data unique to rosiglitazone?

The problem highlighted by rosiglitazone is a system problem – the shortcomings of our national post-approval drug safety surveillance.

Post-approval Drug Safety Issues n n As the patient pool becomes much larger, uncommon but serious side effects will be more evident, but only if data is captured High risk populations or special ethnic groups may need special monitoring The longer duration of drug exposure may lead to new risks for the patient and new challenges for surveillance The widespread use of the approved drug is very variable and often substandard

Post-approval Drug Safety Funding at present is problematic: § The FDA is seriously under-funded § The Prescription Drug User Fee Act (PDUFA) is not yet adequate for all needed safety and efficacy studies § There is an inherent conflict of interest when the pharmaceutical company that created the drug provides nearly all of the funds for the postapproval drug safety research and monitoring § Post-approval drug safety funding from major foundations and from NIH, AHRQ, and FDA is very limited

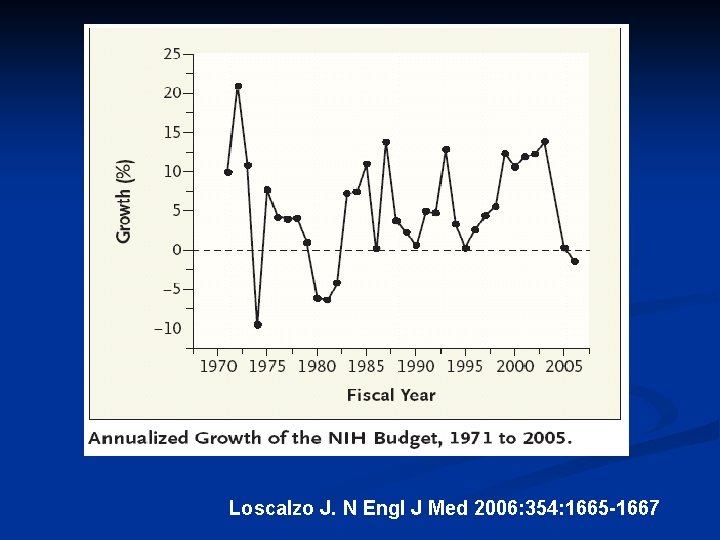
Loscalzo J. N Engl J Med 2006: 354: 1665 -1667

Drug Safety Systems Issues n n n Drug safety is not a given – it is not an inherent feature of most medical systems There is a lack of easy-to-use educational resources on drug safety and efficacy which are available on demand, at the point of care Limited funds to teach all those involved in drug safety as to how to best protect against patient injury The cost of maintaining drug safety is rarely planned for in clinical settings Inadequate electronic information systems for physicians, pharmacists, FDA, and CMS

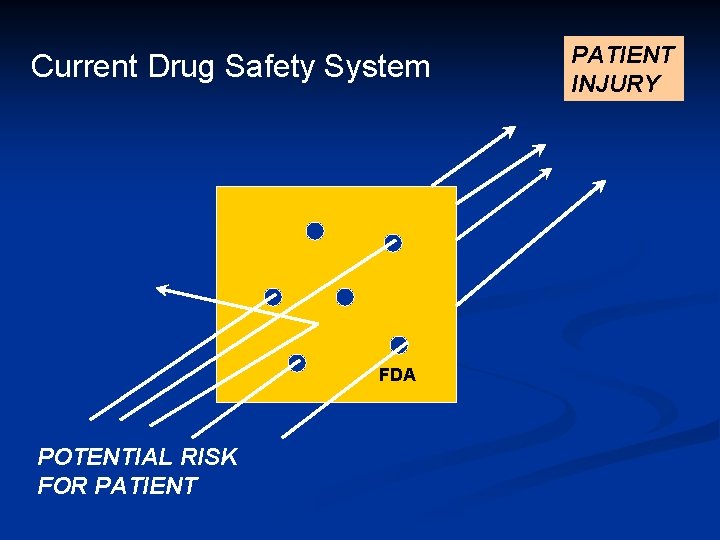
Current Drug Safety System FDA POTENTIAL RISK FOR PATIENT INJURY

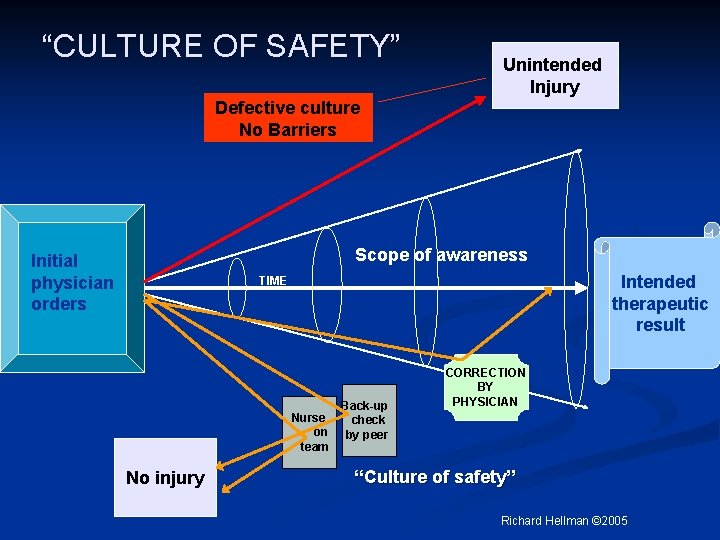
“CULTURE OF SAFETY” Unintended Injury Defective culture No Barriers Scope of awareness Initial physician orders Intended therapeutic result TIME Nurse on team No injury Back-up check by peer CORRECTION BY PHYSICIAN “Culture of safety” Richard Hellman © 2005

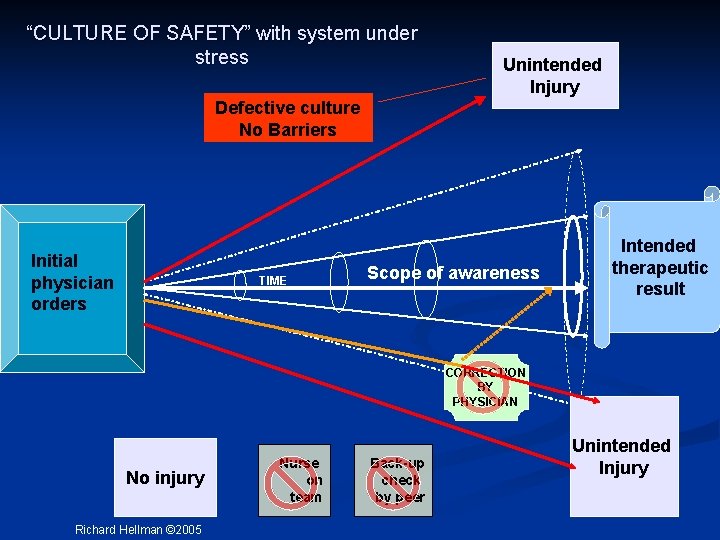
“CULTURE OF SAFETY” with system under stress Unintended Injury Defective culture No Barriers Initial physician orders TIME Scope of awareness Intended therapeutic result CORRECTION BY PHYSICIAN No injury Richard Hellman © 2005 Nurse on team Back-up check by peer Unintended Injury

Drug Safety Data collection is inadequate n The power of informatics to provide high quality epidemiologic data is hampered by the small number of medical centers and physicians using EMR’s n The forms provided for reporting data are impractical and are seriously underutilized n

Cognitive Issues Interfere With Drug Safety n n n Patients frequently neither know what they are taking nor how best to use it safely Physicians and allied health professionals often are not knowledgeable about the medications they use in practice and their hazards Medication reconciliation is seldom done well – often, at the most crucial moment, no one is fully aware of what has or has not been taken by the patient

Proposed Solutions n n n Make drug safety a collective responsibility Funding should be from industry, government and foundations Increase FDA funding for post-approval drug safety Increased responsibility and budget for NIH and AHRQ for post-approval drug safety research RTC’s, casecontrol and epidemiologic studies Increased involvement of physician groups, patient groups and allied health groups in attaining and maintaining drug safety Robust information technology systems to support such an effort

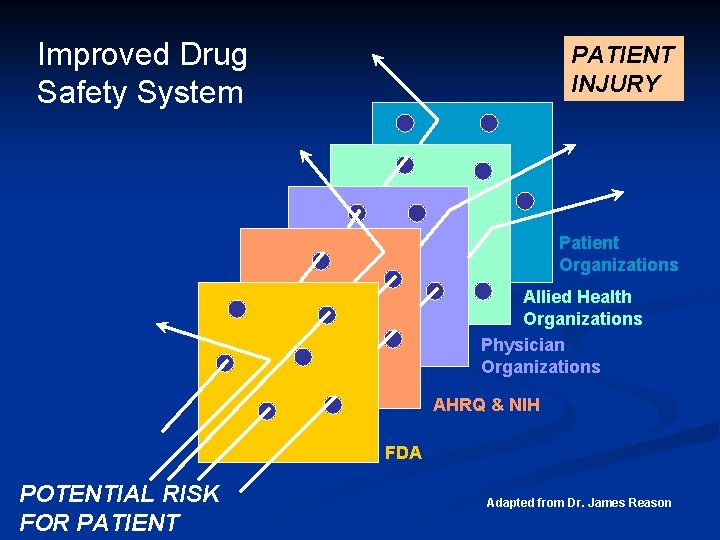
Improved Drug Safety System PATIENT INJURY Patient Organizations Allied Health Organizations Physician Organizations AHRQ & NIH FDA POTENTIAL RISK FOR PATIENT Adapted from Dr. James Reason
 Safetymri
Safetymri Exhausted drug
Exhausted drug Drug and alcohol safety training
Drug and alcohol safety training Chapter 42 hearing speech and vision problems
Chapter 42 hearing speech and vision problems Hearing and equilibrium
Hearing and equilibrium Middle ear
Middle ear Sight hearing taste smell and touch
Sight hearing taste smell and touch Family
Family Proxemics in communication skills
Proxemics in communication skills Hku speech and hearing
Hku speech and hearing Fda v brown and williamson
Fda v brown and williamson Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em