Enzyme Action Enzymes proteins with catalytic function possess
- Slides: 13

Enzyme Action Enzymes … proteins with catalytic function … possess 1 O, 2 O, 3 O and 4 O structure … perform a variety of reactions e. g. , hydrolysis, hydroxylation, oxidation, reduction, transfer of groups … … increase the rate of reaction (up to 1017 -fold) MEDC 527 Fall 2008 1

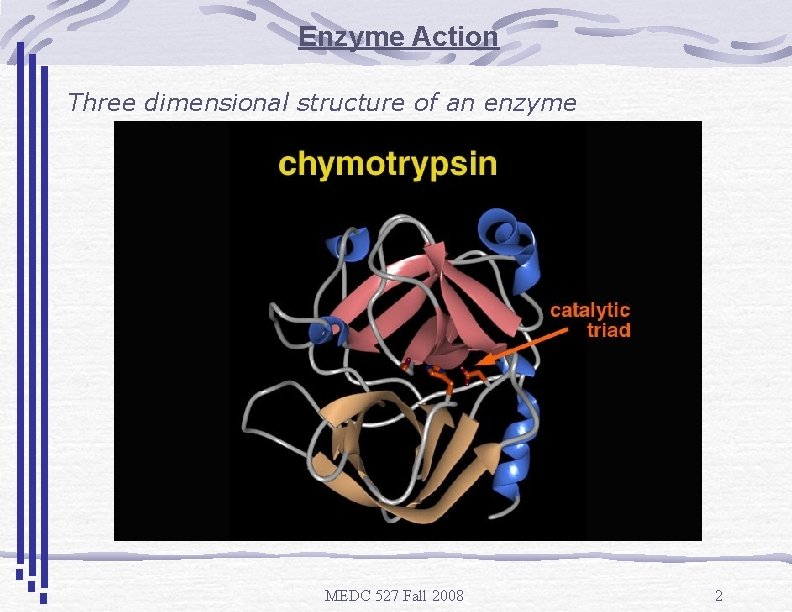
Enzyme Action Three dimensional structure of an enzyme MEDC 527 Fall 2008 2

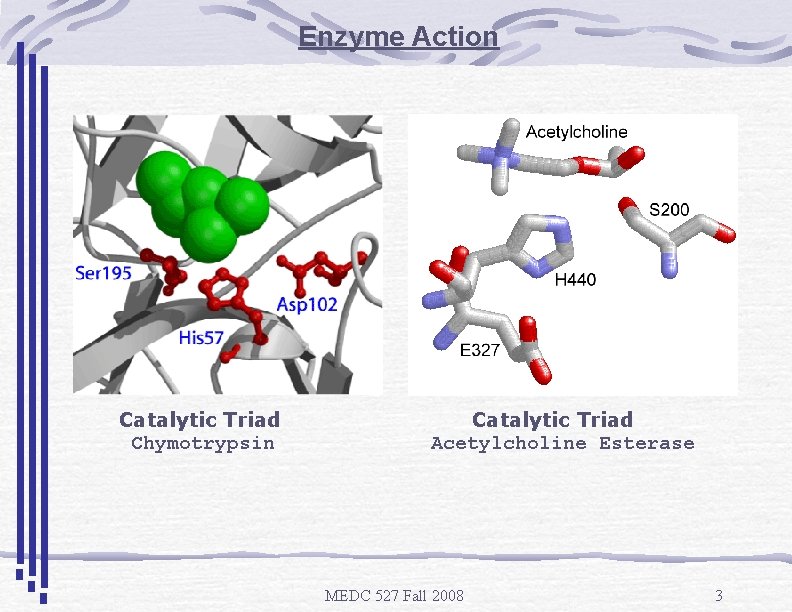
Enzyme Action Catalytic Triad Chymotrypsin Catalytic Triad Acetylcholine Esterase MEDC 527 Fall 2008 3

Chemical Mechanism of Enzyme Action MEDC 527 Fall 2008 4

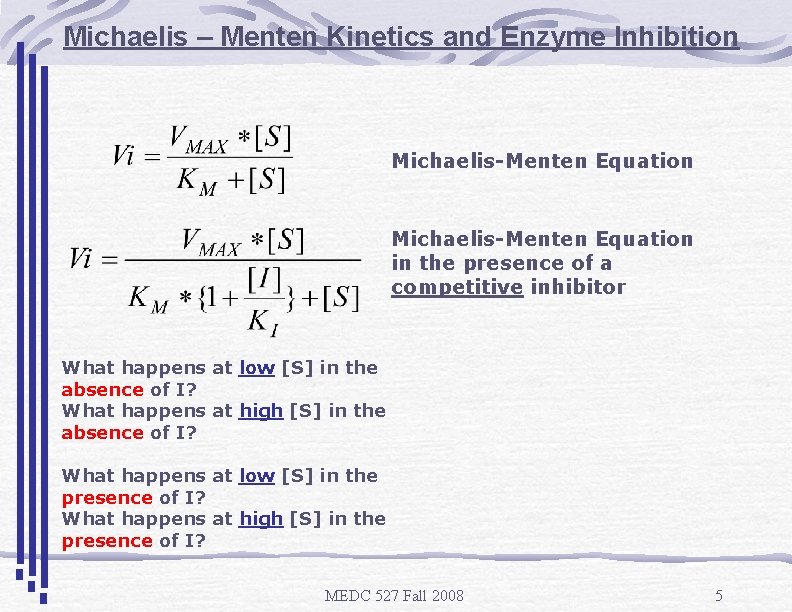
Michaelis – Menten Kinetics and Enzyme Inhibition Michaelis-Menten Equation in the presence of a competitive inhibitor What happens at low [S] in the absence of I? What happens at high [S] in the absence of I? What happens at low [S] in the presence of I? What happens at high [S] in the presence of I? MEDC 527 Fall 2008 5

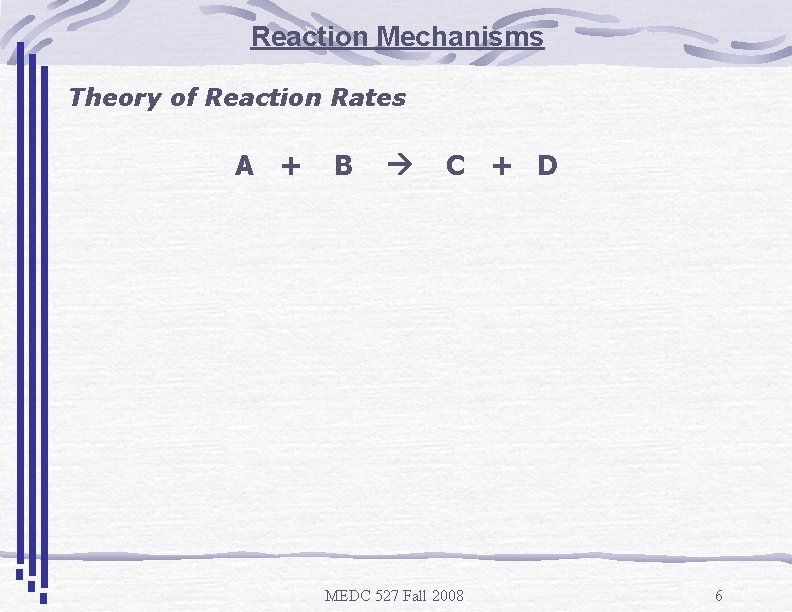
Reaction Mechanisms Theory of Reaction Rates A + B C + D MEDC 527 Fall 2008 6

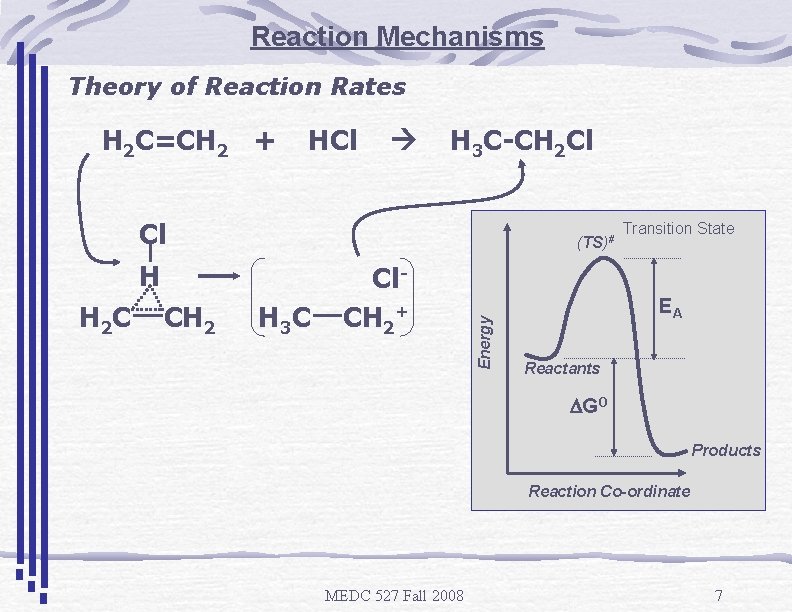
Reaction Mechanisms Theory of Reaction Rates H 2 C=CH 2 + HCl H 3 C-CH 2 Cl Cl H H 2 C CH 2 H 3 C Cl. CH 2+ Energy (TS)# Transition State EA Reactants DGO Products Reaction Co-ordinate MEDC 527 Fall 2008 7

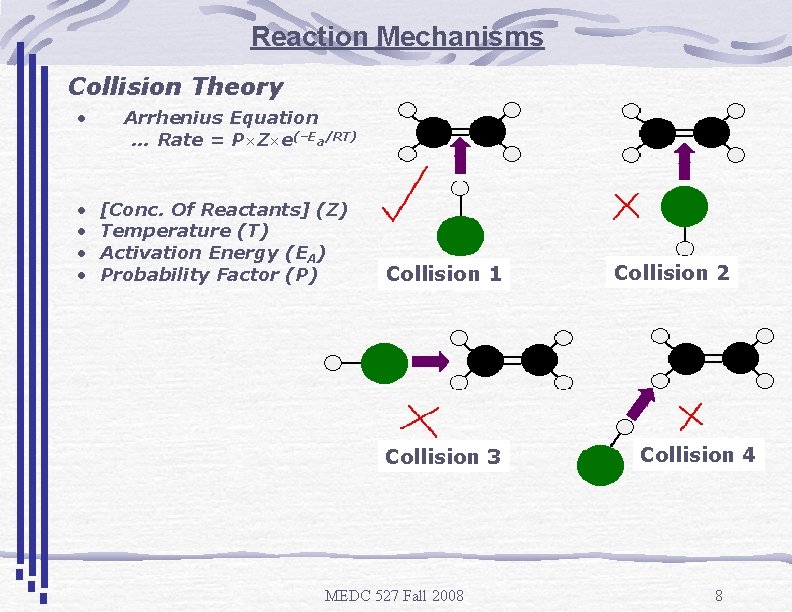
Reaction Mechanisms Collision Theory • • • Arrhenius Equation … Rate = P×Z×e(–Ea/RT) [Conc. Of Reactants] (Z) Temperature (T) Activation Energy (EA) Probability Factor (P) Collision 1 Collision 3 MEDC 527 Fall 2008 Collision 2 Collision 4 8

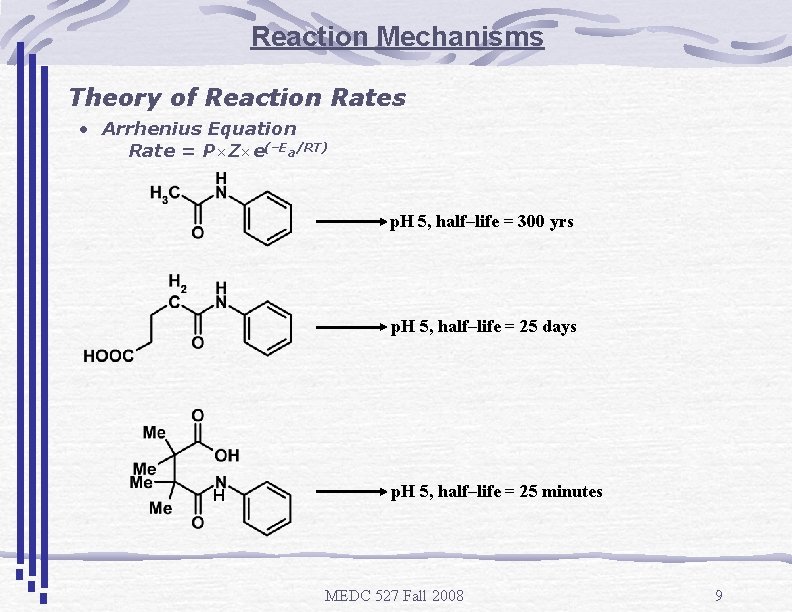
Reaction Mechanisms Theory of Reaction Rates • Arrhenius Equation Rate = P×Z×e(–Ea/RT) p. H 5, half–life = 300 yrs p. H 5, half–life = 25 days H p. H 5, half–life = 25 minutes MEDC 527 Fall 2008 9

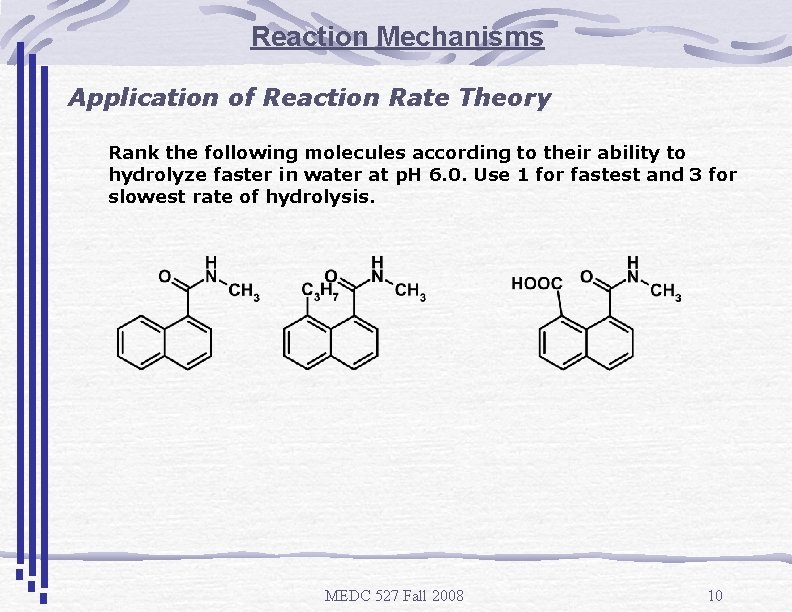
Reaction Mechanisms Application of Reaction Rate Theory Rank the following molecules according to their ability to hydrolyze faster in water at p. H 6. 0. Use 1 for fastest and 3 for slowest rate of hydrolysis. MEDC 527 Fall 2008 10

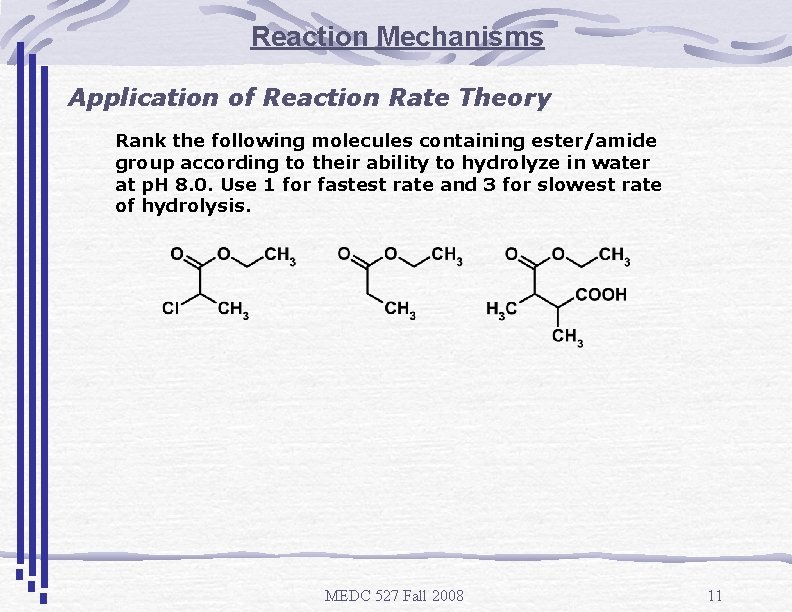
Reaction Mechanisms Application of Reaction Rate Theory Rank the following molecules containing ester/amide group according to their ability to hydrolyze in water at p. H 8. 0. Use 1 for fastest rate and 3 for slowest rate of hydrolysis. MEDC 527 Fall 2008 11

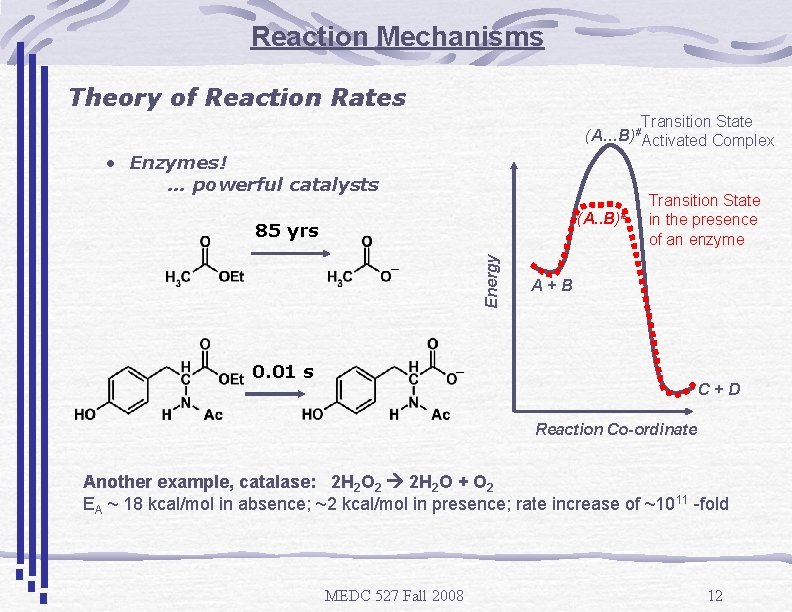
Reaction Mechanisms Theory of Reaction Rates (A…B)# • Enzymes! … powerful catalysts (A. . B)# Energy 85 yrs _ 0. 01 s Transition State Activated Complex Transition State in the presence of an enzyme A+B _ C+D Reaction Co-ordinate Another example, catalase: 2 H 2 O 2 2 H 2 O + O 2 EA ~ 18 kcal/mol in absence; ~2 kcal/mol in presence; rate increase of ~1011 -fold MEDC 527 Fall 2008 12

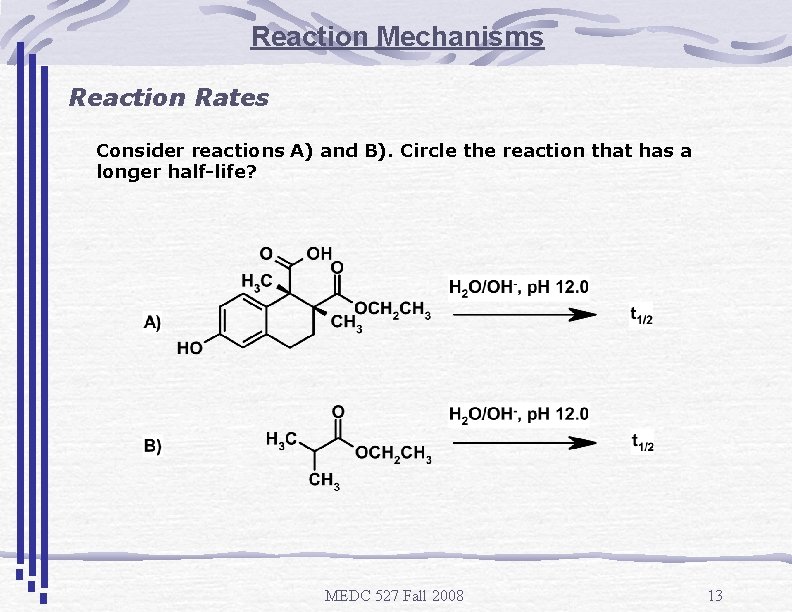
Reaction Mechanisms Reaction Rates Consider reactions A) and B). Circle the reaction that has a longer half-life? MEDC 527 Fall 2008 13